Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 1 Ammonia is a valuable commodity chemical which is produced synthetically by the so-called Haber-Bosch ammonia synthesis reaction: N2(g)+3H2(g)2NH3(g) in a continuous, steady-state industrial
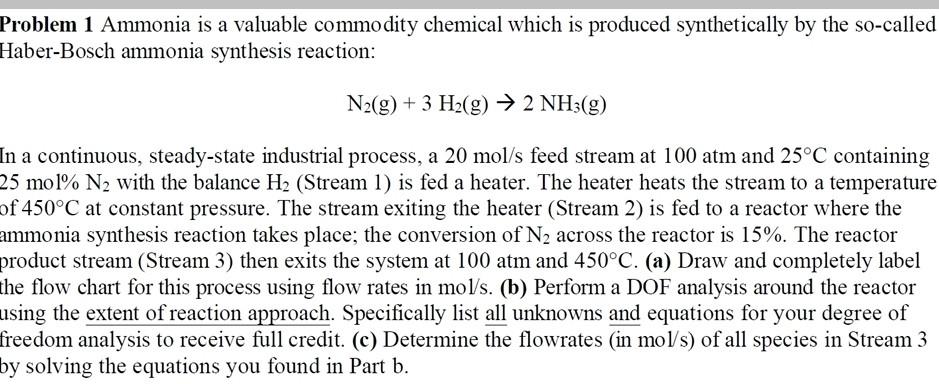
Problem 1 Ammonia is a valuable commodity chemical which is produced synthetically by the so-called Haber-Bosch ammonia synthesis reaction: N2(g)+3H2(g)2NH3(g) in a continuous, steady-state industrial process, a 20mol/s feed stream at 100 atm and 25C containing 25mol2N2 with the balance H2 (Stream 1) is fed a heater. The heater heats the stream to a temperature f 450C at constant pressure. The stream exiting the heater (Stream 2 ) is fed to a reactor where the mmonia synthesis reaction takes place; the conversion of N2 across the reactor is 15%. The reactor product stream (Stream 3) then exits the system at 100 atm and 450C. (a) Draw and completely label he flow chart for this process using flow rates in mol/s. (b) Perform a DOF analysis around the reactor ising the extent of reaction approach. Specifically list all unknowns and equations for your degree of rreedom analysis to receive full credit. (c) Determine the flowrates (in mol/s ) of all species in Stream 3 y solving the equations you found in Part b
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


