Answered step by step
Verified Expert Solution
Question
1 Approved Answer
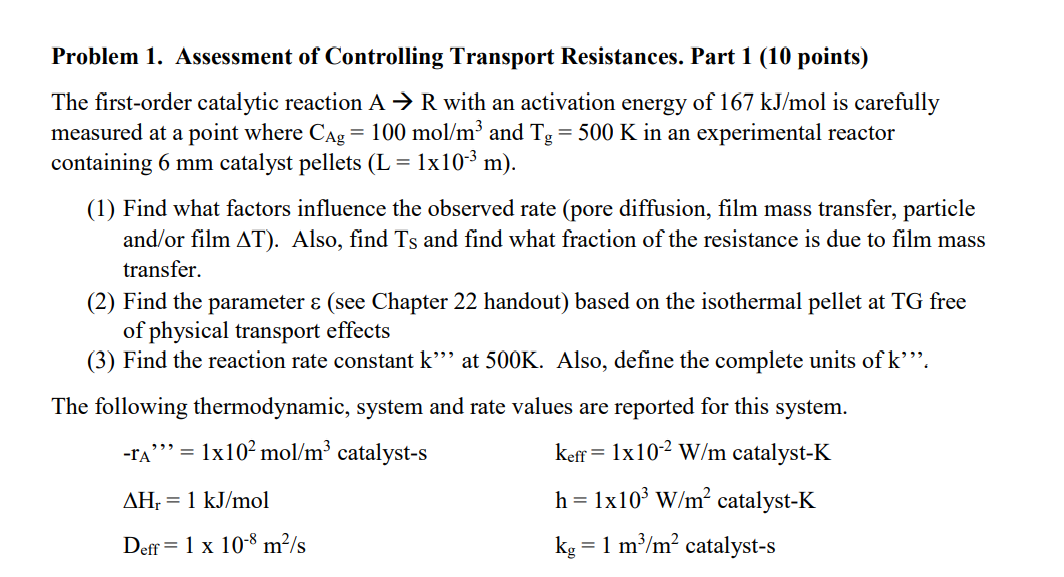
Problem 1 . Assessment of Controlling Transport Resistances. Part 1 ( 1 0 points ) The first - order catalytic reaction A R with an
Problem Assessment of Controlling Transport Resistances. Part points
The firstorder catalytic reaction with an activation energy of is carefully
measured at a point where and in an experimental reactor
containing catalyst pellets
Find what factors influence the observed rate pore diffusion, film mass transfer, particle
andor film Also, find and find what fraction of the resistance is due to film mass
transfer.
Find the parameter see Chapter handout based on the isothermal pellet at TG free
of physical transport effects
Find the reaction rate constant at Also, define the complete units of
The following thermodynamic, system and rate values are reported for this system.
catalyst catalyst
catalyst
catalyst
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


