Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 1: idealised Lenoir heat engine cycle [15 marks] (a) Please explain what adiabatic process, isochoric process and isobaric process are. (b) In an
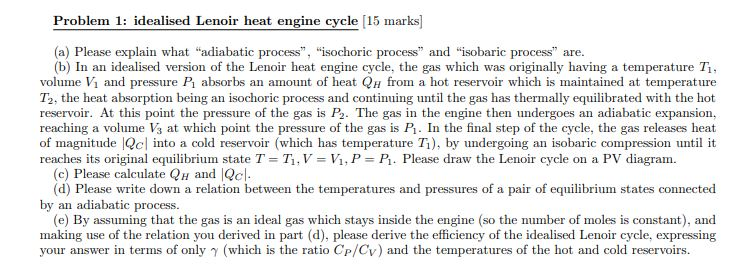
Problem 1: idealised Lenoir heat engine cycle [15 marks] (a) Please explain what "adiabatic process", "isochoric process" and "isobaric process" are. (b) In an idealised version of the Lenoir heat engine cycle, the gas which was originally having a temperature T, volume V and pressure P absorbs an amount of heat QH from a hot reservoir which is maintained at temperature T2, the heat absorption being an isochoric process and continuing until the gas has thermally equilibrated with the hot reservoir. At this point the pressure of the gas is P. The gas in the engine then undergoes an adiabatic expansion, reaching a volume V3 at which point the pressure of the gas is P. In the final step of the cycle, the gas releases heat of magnitude Qc into a cold reservoir (which has temperature 7), by undergoing an isobaric compression until it reaches its original equilibrium state T = T, V = V, P = P. Please draw the Lenoir cycle on a PV diagram. (c) Please calculate QH and Qc. (d) Please write down a relation between the temperatures and pressures of a pair of equilibrium states connected by an adiabatic process. (e) By assuming that the gas is an ideal gas which stays inside the engine (so the number of moles is constant), and making use of the relation you derived in part (d), please derive the efficiency of the idealised Lenoir cycle, expressing your answer in terms of only (which is the ratio Cp/Cy) and the temperatures of the hot and cold reservoirs.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


