Question
Problem 1. One of the mechanisms of atom and radical recombination, called the energy transfer mechanism, is described by the following chemical steps: 2R-R
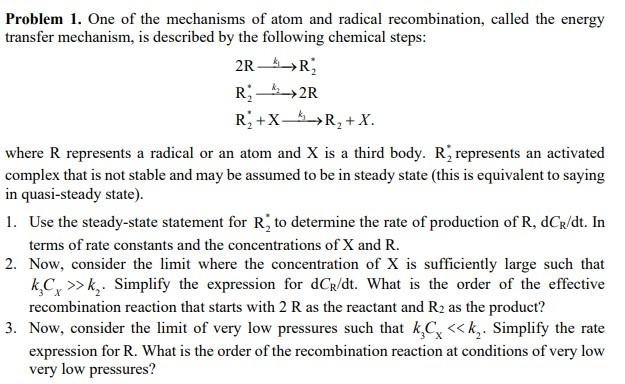
Problem 1. One of the mechanisms of atom and radical recombination, called the energy transfer mechanism, is described by the following chemical steps: 2R-R R2R R +X-R + X. where R represents a radical or an atom and X is a third body. R, represents an activated complex that is not stable and may be assumed to be in steady state (this is equivalent to saying in quasi-steady state). 1. Use the steady-state statement for R, to determine the rate of production of R, dCr/dt. In terms of rate constants and the concentrations of X and R. 2. Now, consider the limit where the concentration of X is sufficiently large such that kC>>k. Simplify the expression for dCR/dt. What is the order of the effective recombination reaction that starts with 2 R as the reactant and R as the product? 3. Now, consider the limit of very low pressures such that kC
Step by Step Solution
3.40 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Molecular Cell Biology
Authors: Harvey Lodish, Arnold Berk, Chris A. Kaiser, Monty Krieger, Anthony Bretscher, Hidde Ploegh, Angelika Amon, Matthew P. Scott
7th edition
1464183393, 1464183392, 978-1429234139
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



