Answered step by step
Verified Expert Solution
Question
1 Approved Answer
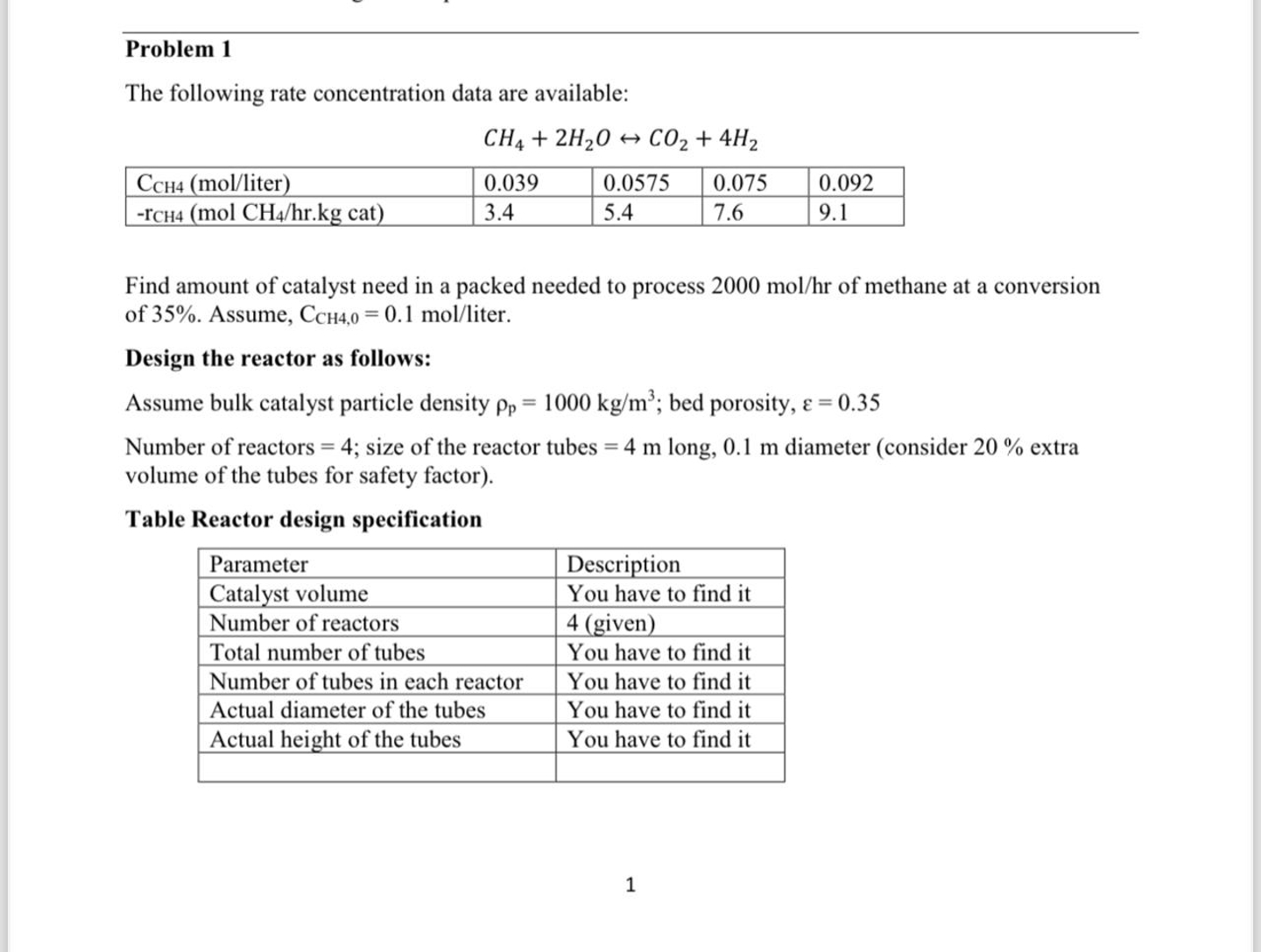
Problem 1 The following rate concentration data are available: Find amount of catalyst need in a packed needed to process 2 0 0 0 m
Problem
The following rate concentration data are available:
Find amount of catalyst need in a packed needed to process of methane at a conversion
of Assume, liter.
Design the reactor as follows:
Assume bulk catalyst particle density ; bed porosity,
Number of reactors ; size of the reactor tubes long, diameter consider extra
volume of the tubes for safety factor
Table Reactor design specification
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


