Question
Problem 2 (30/100): For the following reaction it can be assumed that the reaction heat is constant over the 298-600 K temperature range: NO(g)
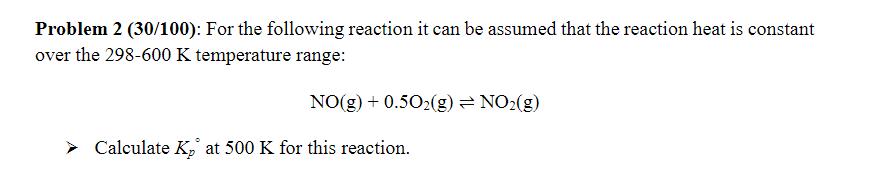
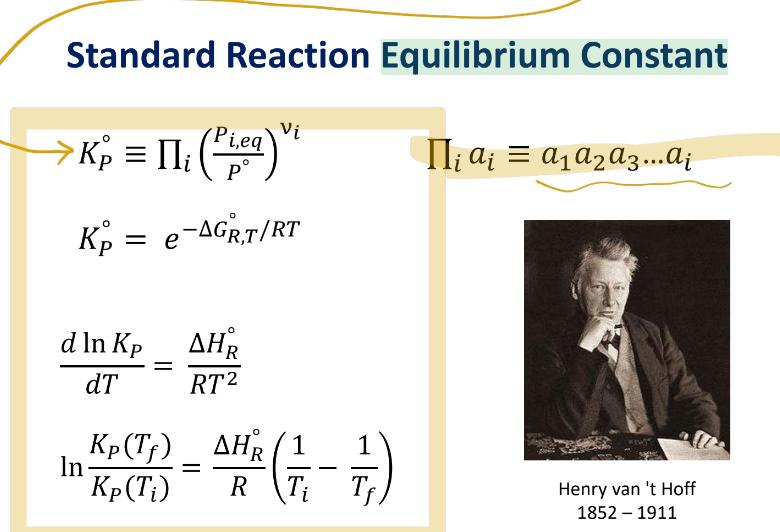
Problem 2 (30/100): For the following reaction it can be assumed that the reaction heat is constant over the 298-600 K temperature range: NO(g) +0.502(g) = NO2(g) Calculate K at 500 K for this reaction. Standard Reaction Equilibrium Constant > [li K = II (PLea) Vi i,eq i a = a2a3...ai Kp = e-AGR,T/RT d In Kp AHR dT RT2 Kp(Tf) In = Kp (Ti) AHR (1 R Ti 1 Tf Henry van 't Hoff 1852-1911
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Transport Operations
Authors: Allen Stuart
2nd Edition
978-0470115398, 0470115394
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



