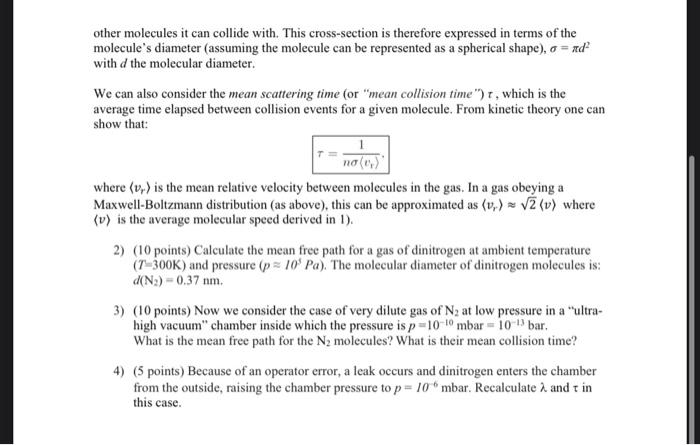
Problem 2 [35 points] - Molecular collisions in nitrogen gas Review the lectures on kinetic theory of gases. We consider a gas of particules (molecules) of mass m whose probability distribution of molecular speeds (v) is given by the MaxwellBoltzmann distribution, also sometimes called a Maxwellian distribution: f(v)dv=4(2kBTm)3/2v2dvemv2/2kBT. where v is the molecular speed, v=v. 1) (10 points) Show that the average molecular speed in the gas is given by: v=m8kBT and calculate v for dinitrogen at T=300K (the mass of a nitrogen atom is 14.007 in atomic mass units, and a dinitrogen molecule is double this). (Hint: derive this result by performing a similar integration procedure as we did in class, see also notes). Note that this quantity v is slightly less than the root-mean-square (rms) speed we derived in class vq=vrms=v2. You can visualize these quantities and shape of the MaxwellBoltzmann distribution on this plot ( vmax is the most likely speed, yet another slightly different value): We are now wanting to investigate "how often" molecules tend to bump into each other in a gas. We define the mean-free-path ( ) for the molecules in a gas as the average distance a molecule travel between collisions with other molecules. Within the kinetic theory of gases, one can show that: =2n1 where n is the number of molecules per unit volume (!not the number of moles!), and is the socalled "collision cross-section" that represents the effective area that each molecule represents to other molecules it can collide with. This cross-section is therefore expressed in terms of the molecule's diameter (assuming the molecule can be represented as a spherical shape), =d2 with d the molecular diameter. We can also consider the mean scattering time (or "mean collision time") , which is the average time elapsed between collision events for a given molecule. From kinetic theory one can show that: =nvr1. where vr is the mean relative velocity between molecules in the gas. In a gas obeying a Maxwell-Boltzmann distribution (as above), this can be approximated as vr2v where v is the average molecular speed derived in 1). 2) (10 points) Calculate the mean free path for a gas of dinitrogen at ambient temperature (T=300K) and pressure (p105Pa). The molecular diameter of dinitrogen molecules is: d(N2)=0.37nm. 3) (10 points) Now we consider the case of very dilute gas of N2 at low pressure in a "ultrahigh vacuum" chamber inside which the pressure is p=1010mbar=1013 bar. What is the mean free path for the N2 molecules? What is their mean collision time? 4) (5 points) Because of an operator error, a leak occurs and dinitrogen enters the chamber from the outside, raising the chamber pressure to p=106mbar. Recalculate and in this case