Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 2 (40 points) Situation: This problem deals with an ideal, monoatomic gas [Cp=(5/2)R]. This gas is initially at 0.10 [MPa] and occupies a volu
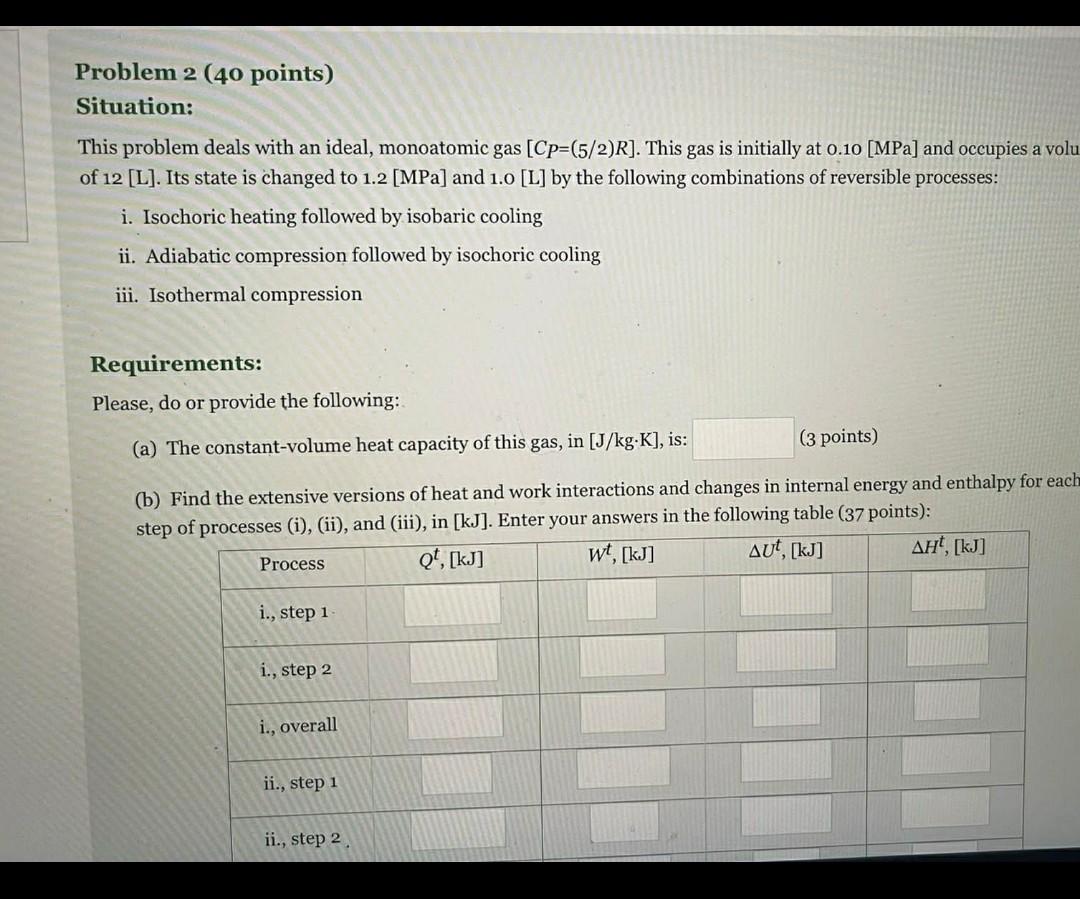
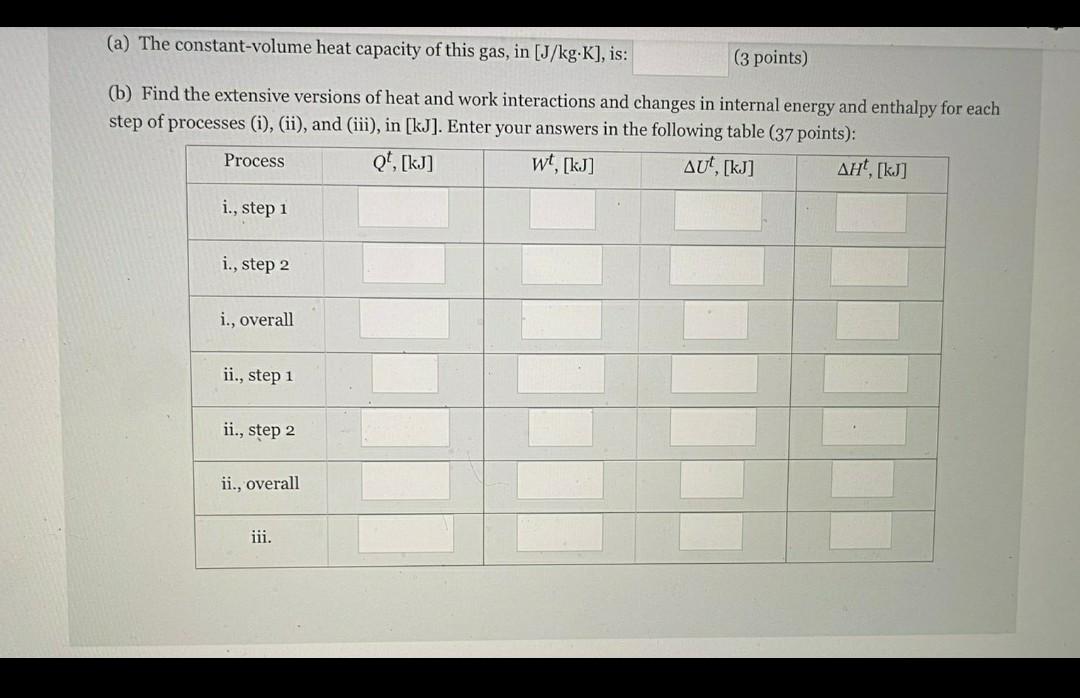
Problem 2 (40 points) Situation: This problem deals with an ideal, monoatomic gas [Cp=(5/2)R]. This gas is initially at 0.10 [MPa] and occupies a volu of 12 [L]. Its state is changed to 1.2 [MPa] and 1.0 [L] by the following combinations of reversible processes: i. Isochoric heating followed by isobaric cooling ii. Adiabatic compression followed by isochoric cooling iii. Isothermal compression Requirements: Please, do or provide the following: (a) The constant-volume heat capacity of this gas, in [J/kg.K], is: (3 points) (b) Find the extensive versions of heat and work interactions and changes in internal energy and enthalpy for each step of processes (i), (ii), and (iii), in [kJ]. Enter your answers in the following table (37 points): wt, [kj] t, [kg] Qt, [kj] AUT, [kj] Process i., step 1 i., step 2 i., overall ii., step 1 ii., step 2 (a) The constant-volume heat capacity of this gas, in [J/kg.K], is: (3 points) (b) Find the extensive versions of heat and work interactions and changes in internal energy and enthalpy for each step of processes (i), (ii), and (iii), in [kJ]. Enter your answers in the following table (37 points): Qt. [kj] Process wt, [kj] AUT, [kj] , [kJ] i., step 1 i., step 2 i., overall ii., step 1 ii., step 2 ii., overall iii. Problem 2 (40 points) Situation: This problem deals with an ideal, monoatomic gas [Cp=(5/2)R]. This gas is initially at 0.10 [MPa] and occupies a volu of 12 [L]. Its state is changed to 1.2 [MPa] and 1.0 [L] by the following combinations of reversible processes: i. Isochoric heating followed by isobaric cooling ii. Adiabatic compression followed by isochoric cooling iii. Isothermal compression Requirements: Please, do or provide the following: (a) The constant-volume heat capacity of this gas, in [J/kg.K], is: (3 points) (b) Find the extensive versions of heat and work interactions and changes in internal energy and enthalpy for each step of processes (i), (ii), and (iii), in [kJ]. Enter your answers in the following table (37 points): wt, [kj] t, [kg] Qt, [kj] AUT, [kj] Process i., step 1 i., step 2 i., overall ii., step 1 ii., step 2 (a) The constant-volume heat capacity of this gas, in [J/kg.K], is: (3 points) (b) Find the extensive versions of heat and work interactions and changes in internal energy and enthalpy for each step of processes (i), (ii), and (iii), in [kJ]. Enter your answers in the following table (37 points): Qt. [kj] Process wt, [kj] AUT, [kj] , [kJ] i., step 1 i., step 2 i., overall ii., step 1 ii., step 2 ii., overall
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


