Answered step by step
Verified Expert Solution
Question
1 Approved Answer
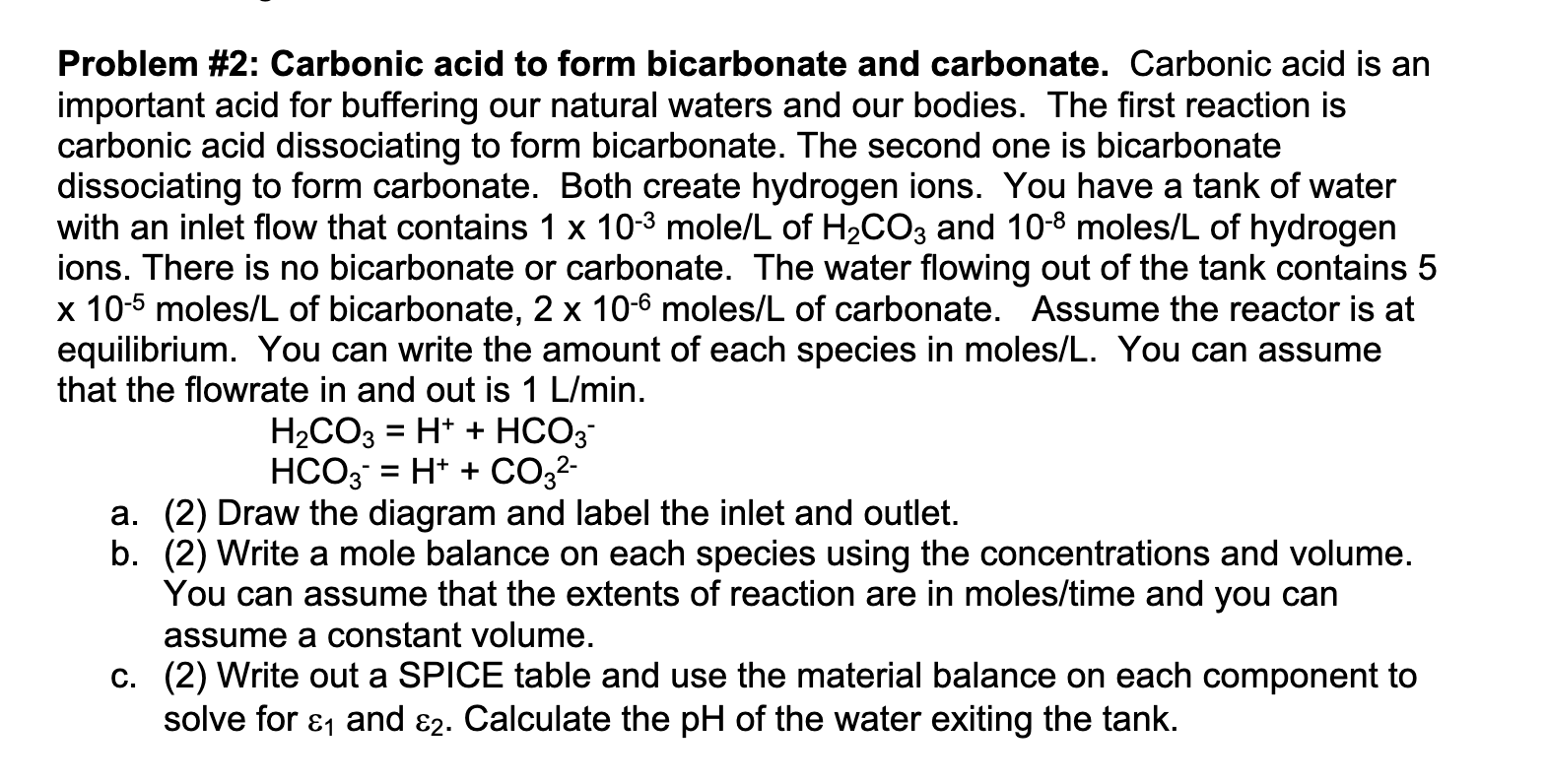
Problem # 2 : Carbonic acid to form bicarbonate and carbonate. Carbonic acid is an important acid for buffering our natural waters and our bodies.
Problem #: Carbonic acid to form bicarbonate and carbonate. Carbonic acid is an
important acid for buffering our natural waters and our bodies. The first reaction is
carbonic acid dissociating to form bicarbonate. The second one is bicarbonate
dissociating to form carbonate. Both create hydrogen ions. You have a tank of water
with an inlet flow that contains mole and mole of hydrogen
ions. There is no bicarbonate or carbonate. The water flowing out of the tank contains
moles of bicarbonate, moles of carbonate. Assume the reactor is at
equilibrium. You can write the amount of each species in molesL You can assume
that the flowrate in and out is
a Draw the diagram and label the inlet and outlet.
b Write a mole balance on each species using the concentrations and volume.
You can assume that the extents of reaction are in molestime and you can
assume a constant volume.
c Write out a SPICE table and use the material balance on each component to
solve for and Calculate the of the water exiting the tank.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


