Answered step by step
Verified Expert Solution
Question
1 Approved Answer
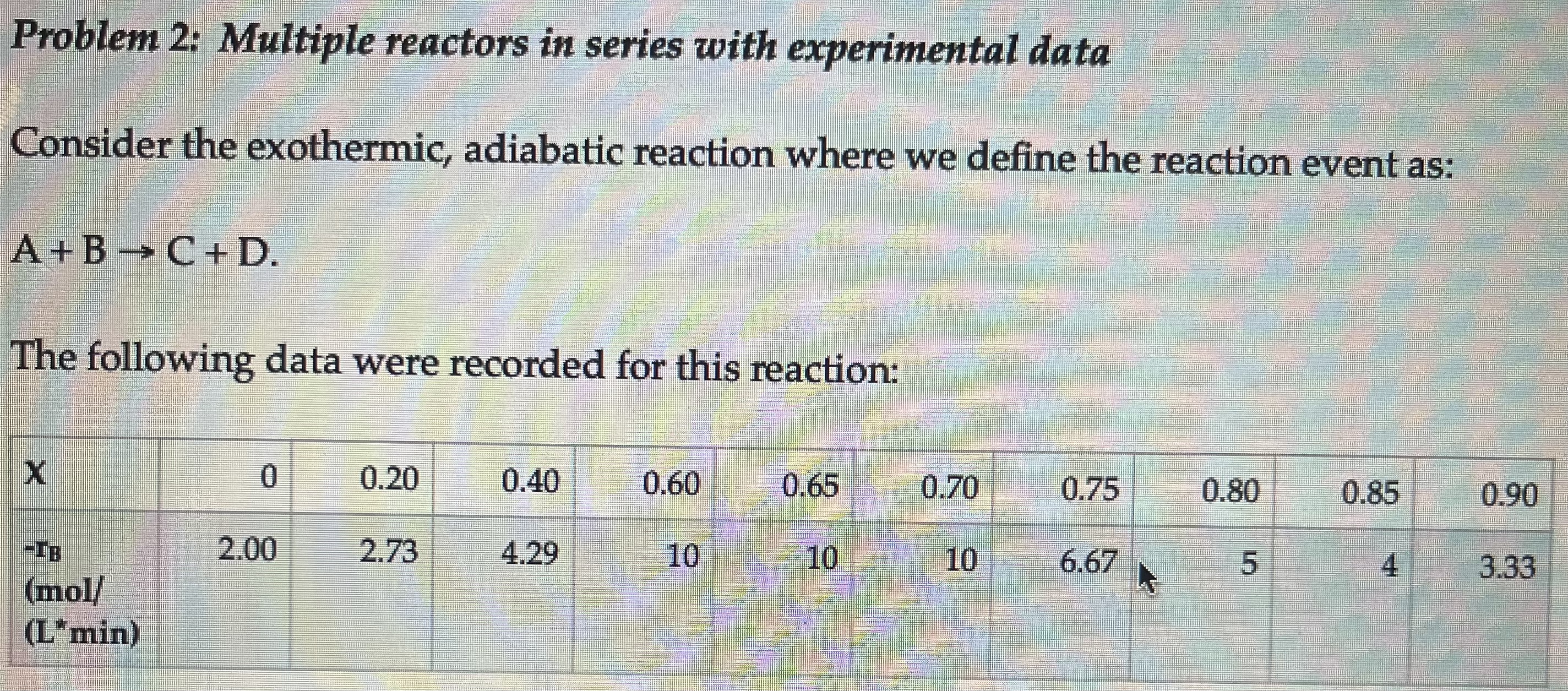
Problem 2 : Multiple reactors in series with experimental data Consider the exothermic, adiabatic reaction where we define the reaction event as: A + B
Problem : Multiple reactors in series with experimental data
Consider the exothermic, adiabatic reaction where we define the reaction event as:
A B C D
The following data were recorded for this reaction:
X
rB
mol
Lmin
The total entering molar flow rate is mols where the reactants are fed in the ratio of
:A:B
Problem : Multiple reactors in series with experimental data
Consider the exothermic, adiabatic reaction where we define the reaction event as:
The following data were recorded for this reaction:c What if you instead begin with a L CSTR and you follow it with a PFR What
volume of a PFR do you need to achieve conversion? L
d Is there a range of conversions over which we can use the same size PFR and
CSTR to achieve the same conversion? If so what is the range?
e To achieve an overall conversion of what series of reactors will minimize
the total required volume?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


