Answered step by step
Verified Expert Solution
Question
1 Approved Answer
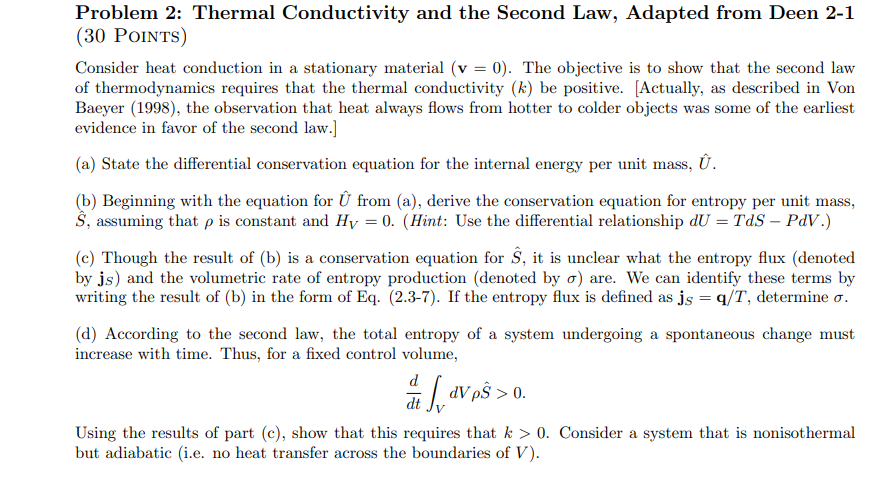
Problem 2 : Thermal Conductivity and the Second Law, Adapted from Deen 2 - 1 ( 3 0 Points ) Consider heat conduction in a
Problem : Thermal Conductivity and the Second Law, Adapted from Deen
Points
Consider heat conduction in a stationary material The objective is to show that the second law
of thermodynamics requires that the thermal conductivity be positive. Actually as described in Von
Baeyer the observation that heat always flows from hotter to colder objects was some of the earliest
evidence in favor of the second law.
a State the differential conservation equation for the internal energy per unit mass, hat
b Beginning with the equation for hat from a derive the conservation equation for entropy per unit mass,
hat assuming that is constant and Hint: Use the differential relationship
c Though the result of b is a conservation equation for hat it is unclear what the entropy flux denoted
by and the volumetric rate of entropy production denoted by are. We can identify these terms by
writing the result of b in the form of Eq If the entropy flux is defined as determine
d According to the second law, the total entropy of a system undergoing a spontaneous change must
increase with time. Thus, for a fixed control volume,
Using the results of part c show that this requires that Consider a system that is nonisothermal
but adiabatic ie no heat transfer across the boundaries of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


