Question
(a) (i) Derive an expression to calculate entropy change for an ideal gas when the temperature changes from T to T and pressure changes
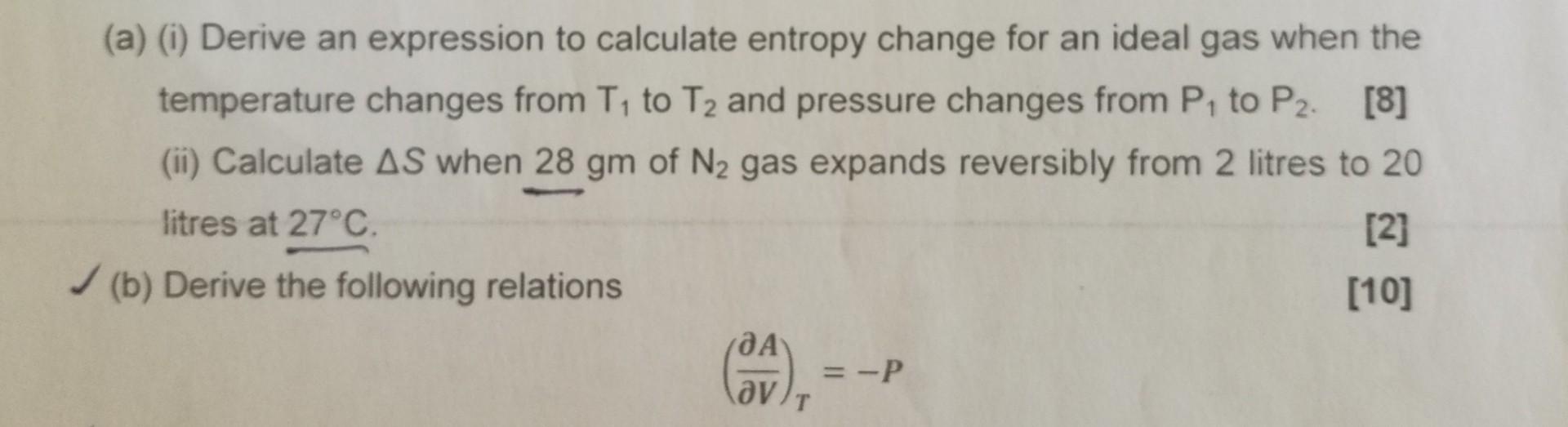
(a) (i) Derive an expression to calculate entropy change for an ideal gas when the temperature changes from T to T and pressure changes from P to P2. [8] (ii) Calculate AS when 28 gm of N gas expands reversibly from 2 litres to 20 litres at 27C. [2] (b) Derive the following relations [10] (JA)), . =-P
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Acc oto Helmholtz fxn da PavSat at const temp Pdv da divide both si...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Advanced Accounting
Authors: Debra C. Jeter, Paul K. Chaney
7th edition
1119373204, 9781119373254 , 978-1119373209
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



