Answered step by step
Verified Expert Solution
Question
1 Approved Answer
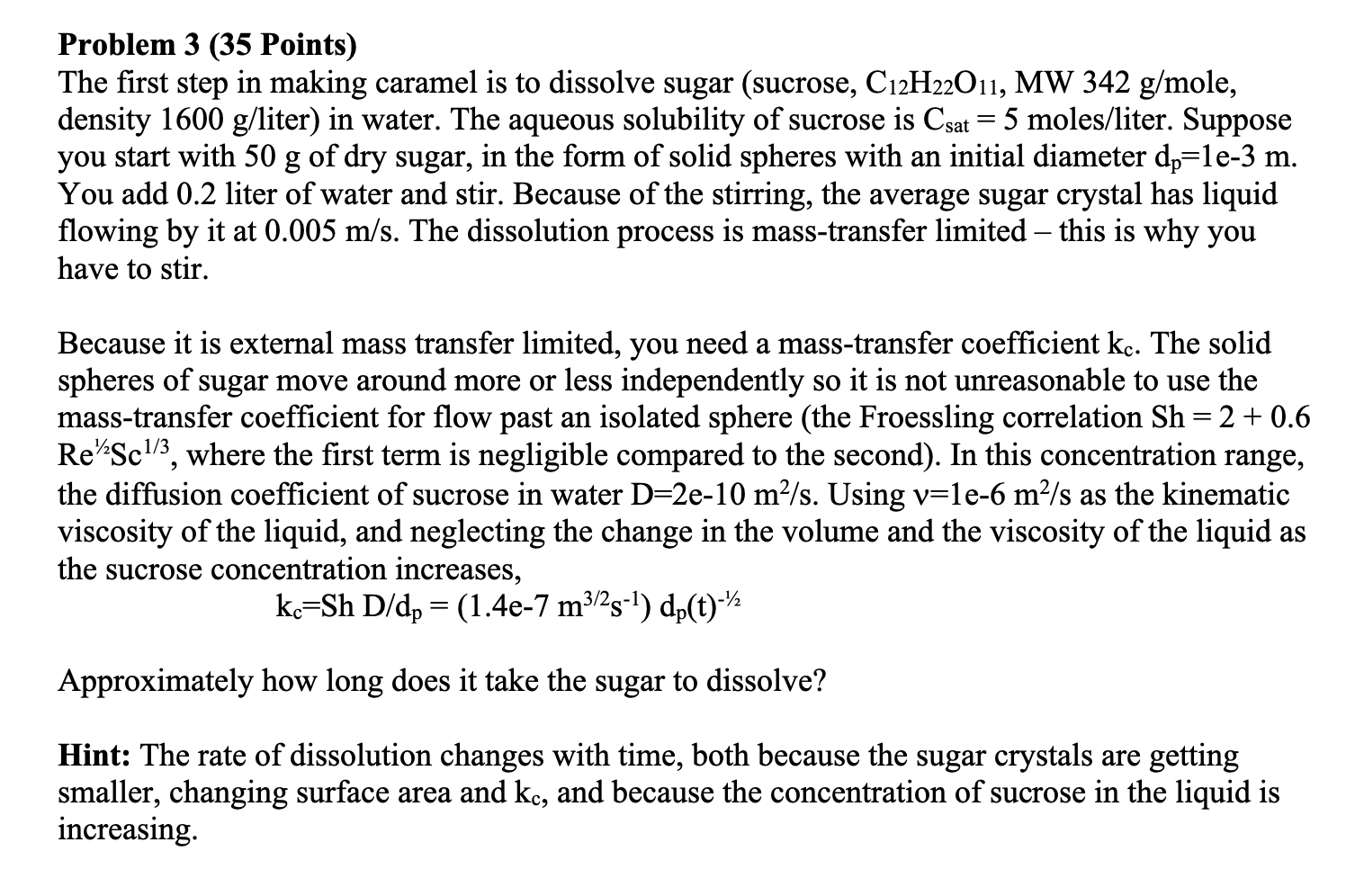
Problem 3 ( 3 5 Points ) The first step in making caramel is to dissolve sugar ( sucrose , C 1 2 H 2
Problem Points
The first step in making caramel is to dissolve sugar sucrose MW ole,
density liter in water. The aqueous solubility of sucrose is mole liter. Suppose
you start with of dry sugar, in the form of solid spheres with an initial diameter
You add liter of water and stir. Because of the stirring, the average sugar crystal has liquid
flowing by it at The dissolution process is masstransfer limited this is why you
have to stir.
Because it is external mass transfer limited you need a masstransfer coefficient The solid
spheres of sugar move around more or less independently so it is not unreasonable to use the
masstransfer coefficient for flow past an isolated sphere the Froessling correlation
where the first term is negligible compared to the second In this concentration range,
the diffusion coefficient of sucrose in water Using as the kinematic
viscosity of the liquid, and neglecting the change in the volume and the viscosity of the liquid as
the sucrose concentration increases,
Approximately how long does it take the sugar to dissolve?
Hint: The rate of dissolution changes with time, both because the sugar crystals are getting
smaller, changing surface area and and because the concentration of sucrose in the liquid is
increasing.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


