Answered step by step
Verified Expert Solution
Question
1 Approved Answer
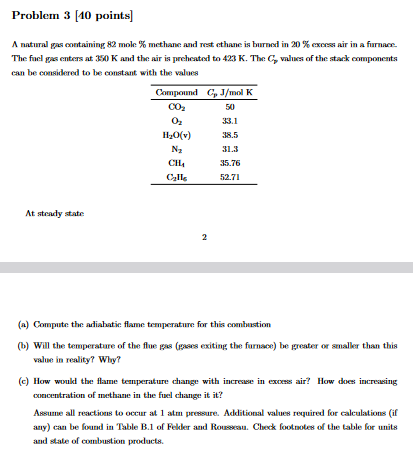
Problem 3 [ 4 0 points ] A natural gas containing 8 2 mole % methane and rest ethane is burned in 2 0 %
Problem points
A natural gas containing mole methane and rest ethane is burned in exers air in a furnax.
The fuel gas enters at and the air is preheated to The values of the stark components
can be considered to be constant, with the values
At strady state
a Compute the adiahatic flame temperature for this combustion
b Will the temperature of the flue gas gases exiting the furnace be greater or analler than this
value in reality? Why?
c How would the flame tenperature change with increase in excess air? How does increasing
concentration of methane in the fuel change it it
Assume all reactions to occur at atm pressure. Additional values required for calculations if
any can be found in Table B of Felder and Rousesan. Check footnoles of the table for units
and stale of combustion products.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


