Answered step by step
Verified Expert Solution
Question
1 Approved Answer
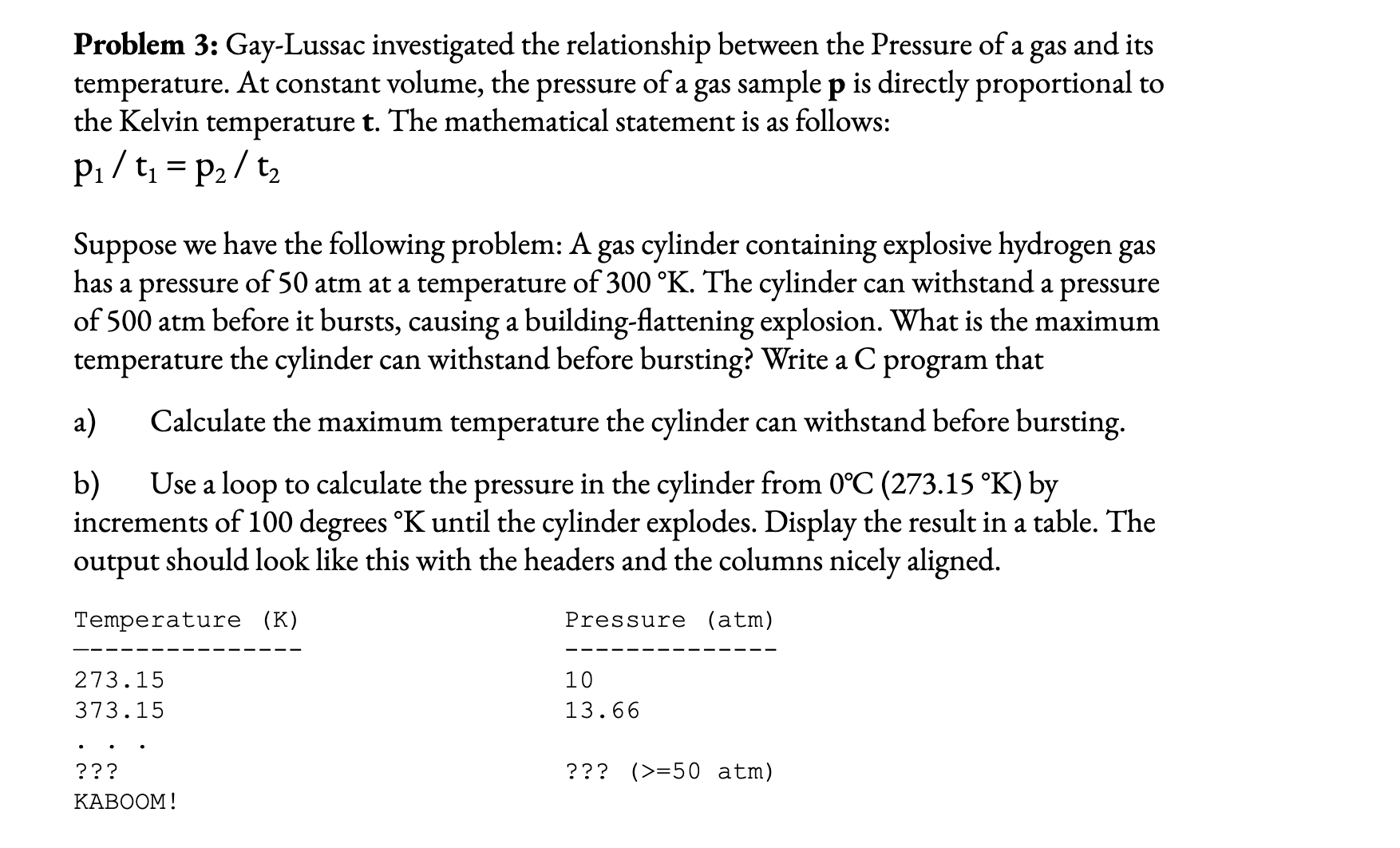
Problem 3 : Gay - Lussac investigated the relationship between the Pressure of a gas and its temperature. At constant volume, the pressure of a
Problem : GayLussac investigated the relationship between the Pressure of a gas and its
temperature. At constant volume, the pressure of a gas sample is directly proportional to
the Kelvin temperature The mathematical statement is as follows:
Suppose we have the following problem: A gas cylinder containing explosive hydrogen gas
has a pressure of atm at a temperature of The cylinder can withstand a pressure
of atm before it bursts, causing a buildingflattening explosion. What is the maximum
temperature the cylinder can withstand before bursting? Write a program that
a Calculate the maximum temperature the cylinder can withstand before bursting.
b Use a loop to calculate the pressure in the cylinder from by
increments of degrees until the cylinder explodes. Display the result in a table. The
output should look like this with the headers and the columns nicely aligned.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


