Answered step by step
Verified Expert Solution
Question
1 Approved Answer
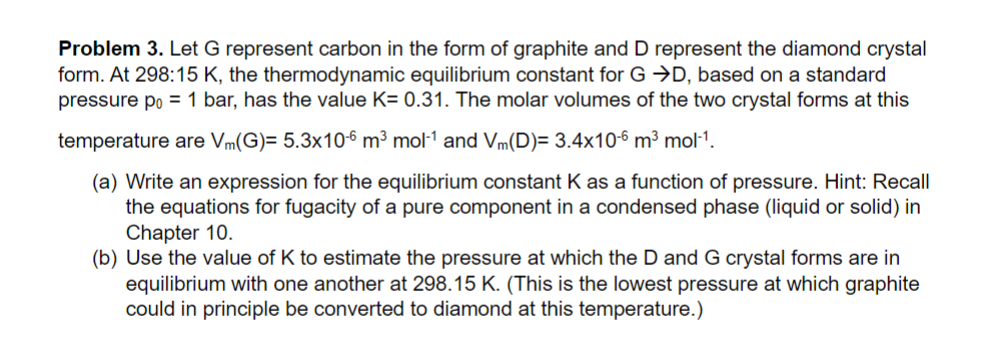
Problem 3 . Let G represent carbon in the form of graphite and D represent the diamond crystal form. At 2 9 8 . 1
Problem Let represent carbon in the form of graphite and represent the diamond crystal form. At K the thermodynamic equilibrium constant for based on a standard pressure bar, has the value The molar volumes of the two crystal forms at this temperature are and
a Write an expression for the equilibrium constant as a function of pressure. Hint: Recall the equations for fugacity of a pure component in a condensed phase liquid or solid in Chapter
b Use the value of to estimate the pressure at which the and crystal forms are in equilibrium with one another at This is the lowest pressure at which graphite could in principle be converted to diamond at this temperature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


