Question
Problem 3. We are planning to operate a batch reactor to convert AR. This is a liquid reaction and the reaction rate data are
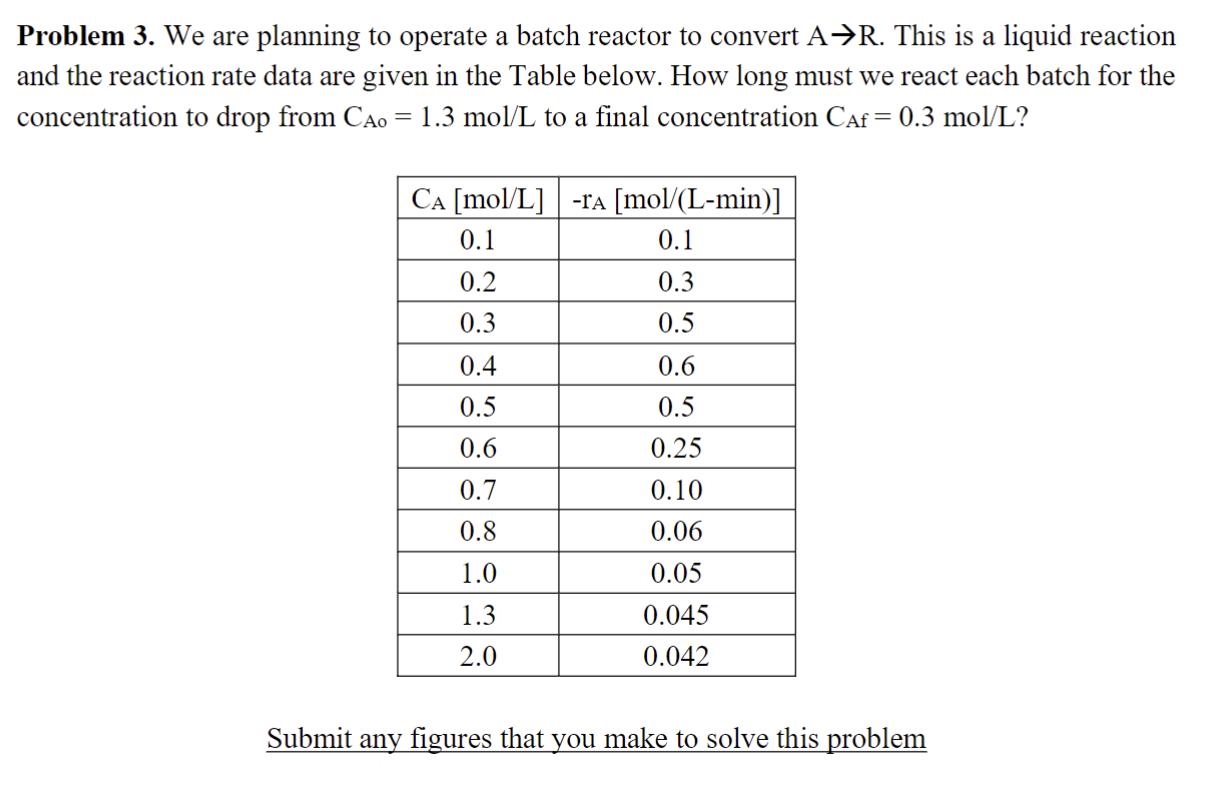
Problem 3. We are planning to operate a batch reactor to convert AR. This is a liquid reaction and the reaction rate data are given in the Table below. How long must we react each batch for the concentration to drop from CA0 = 1.3 mol/L to a final concentration CAf = 0.3 mol/L? CA [mol/L] -TA [mol/(L-min)] 0.1 0.1 0.2 0.3 0.3 0.5 0.4 0.6 0.5 0.5 0.6 0.25 0.7 0.10 0.8 0.06 1.0 0.05 1.3 0.045 2.0 0.042 Submit any figures that you make to solve this problem
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Reaction Engineering
Authors: Octave Levenspiel
3rd Edition
047125424X, 978-0471254249
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



