Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the isomerization of n-butane to iso-butane as a first order gas phase reaction with ideal gas behavior. A rate constant of 0.0015 min
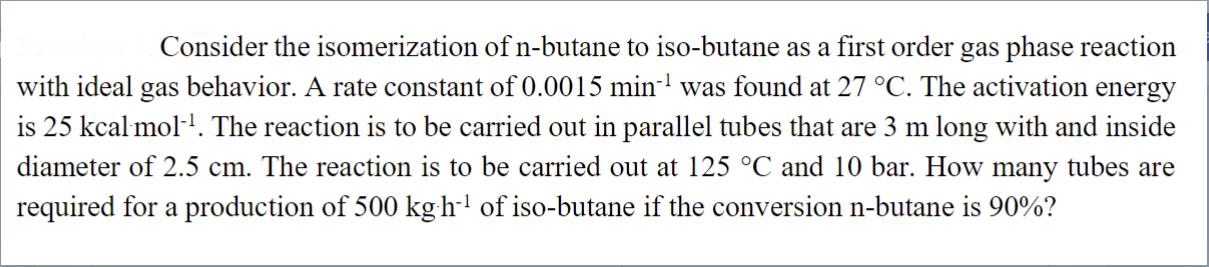
Consider the isomerization of n-butane to iso-butane as a first order gas phase reaction with ideal gas behavior. A rate constant of 0.0015 min was found at 27 C. The activation energy is 25 kcal mol. The reaction is to be carried out in parallel tubes that are 3 m long with and inside diameter of 2.5 cm. The reaction is to be carried out at 125 C and 10 bar. How many tubes are required for a production of 500 kg h of iso-butane if the conversion n-butane is 90%?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this problem we need to first calculate the rate constant at the new temperature of 125C Gi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
663e4d343fefe_958432.pdf
180 KBs PDF File
663e4d343fefe_958432.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


