Question
The following table gives the instantaneous rates of the disappearance of A in a reaction between A and B, at various concentrations of both
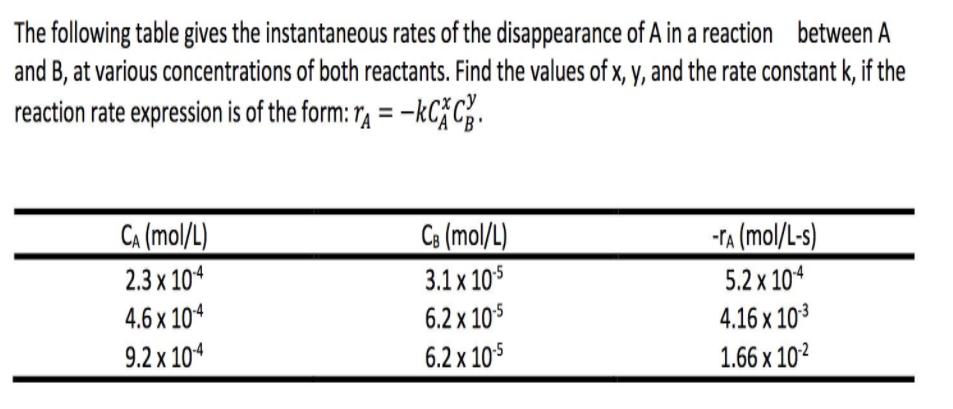
The following table gives the instantaneous rates of the disappearance of A in a reaction between A and B, at various concentrations of both reactants. Find the values of x, y, and the rate constant k, if the reaction rate expression is of the form: 1, = -kC} C. Ce (mol/L) 3.1 x 105 6.2 x 105 CA (mol/L) -TA (mol/L-s) 2.3 x 104 5.2 x 104 4.6 x 104 9.2 x 104 4.16 x 103 1.66 x 102 6.2 x 105
Step by Step Solution
3.35 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
Answer The Reacbien Robe equaticn kC 23 x 10 4 mode 4 TA 5 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Regression Analysis And Other Multivariable Methods
Authors: David G. Kleinbaum, Lawrence L. Kupper, Azhar Nizam, Eli S. Rosenberg
5th Edition
1285051084, 978-1285963754, 128596375X, 978-1285051086
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



