Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 5 The closed, perfectly insulated cylinder shown has circular cross section and has two compartments separated by a movable piston with no heat
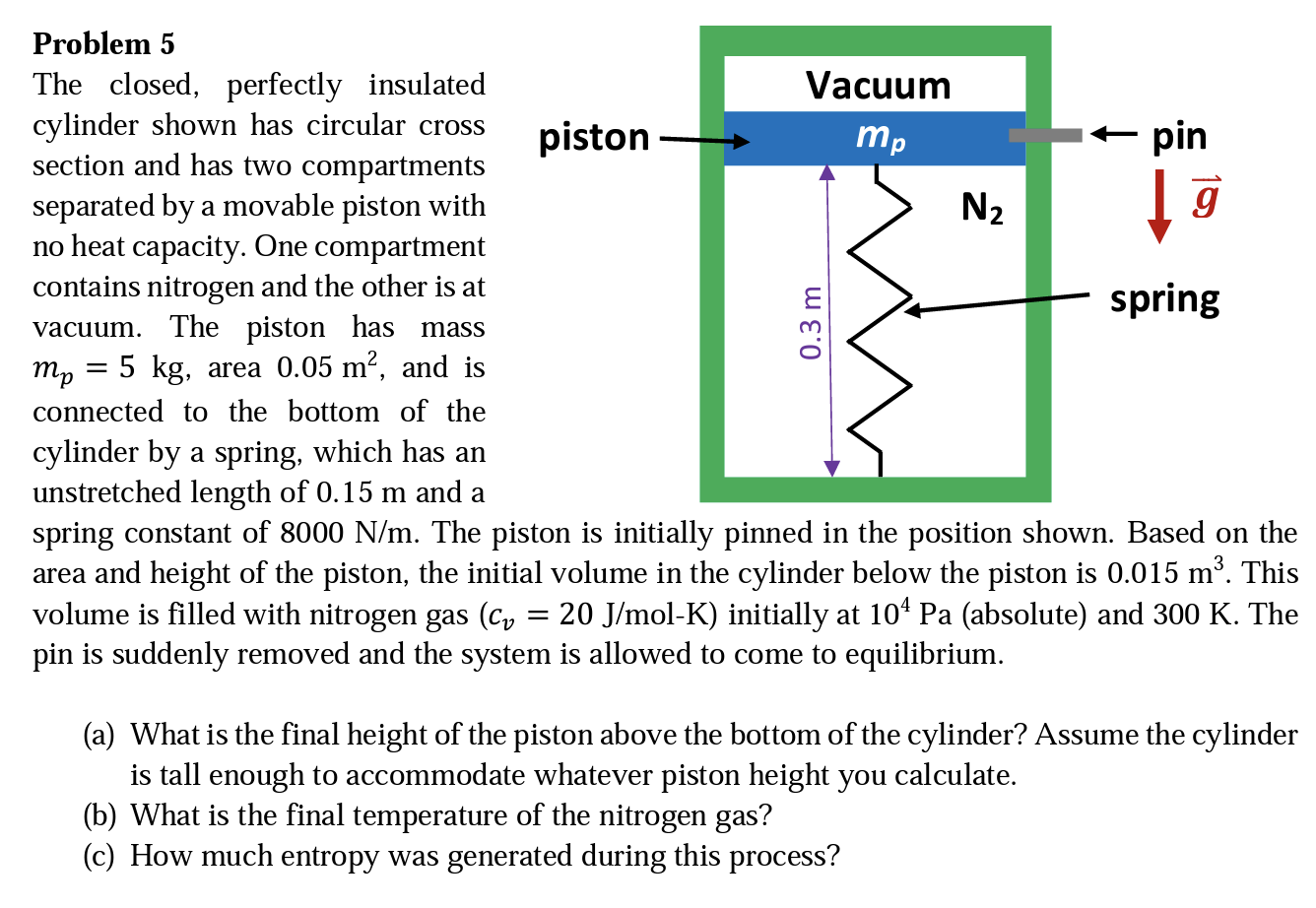
Problem 5 The closed, perfectly insulated cylinder shown has circular cross section and has two compartments separated by a movable piston with no heat capacity. One compartment contains nitrogen and the other is at vacuum. The piston has mass mp = 5 kg, area 0.05 m, and is connected to the bottom of the cylinder by a spring, which has an unstretched length of 0.15 m and a piston Vacuum mp pin N 9 g spring 0.3 m spring constant of 8000 N/m. The piston is initially pinned in the position shown. Based on the area and height of the piston, the initial volume in the cylinder below the piston is 0.015 m. This volume is filled with nitrogen gas (Cv 20 J/mol-K) initially at 104 Pa (absolute) and 300 K. The = pin is suddenly removed and the system is allowed to come to equilibrium. (a) What is the final height of the piston above the bottom of the cylinder? Assume the cylinder is tall enough to accommodate whatever piston height you calculate. (b) What is the final temperature of the nitrogen gas? (c) How much entropy was generated during this process?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


