Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 7-10 (Level 1) An ideal batch reactor is to be sized for the polymerization of styrene monomer to polystyrene. Pure styrene monomer containing
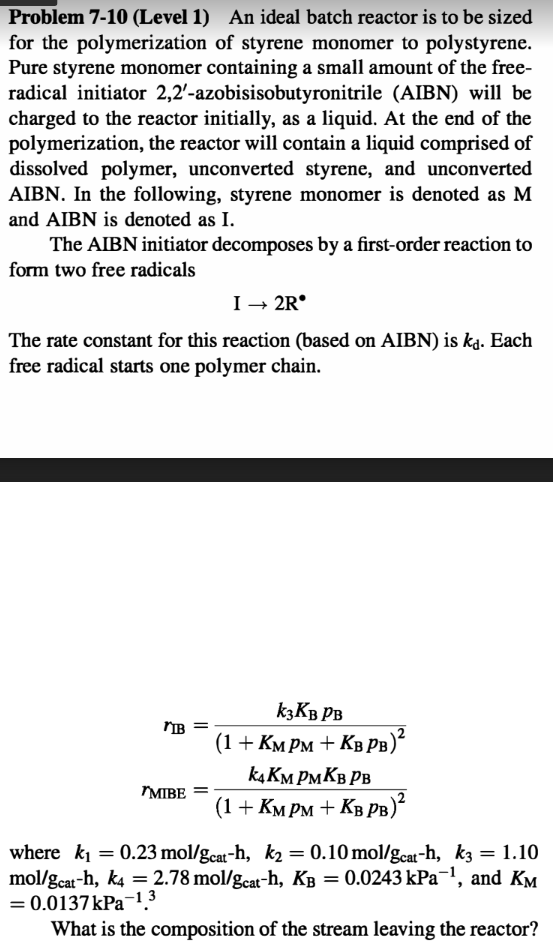
Problem 7-10 (Level 1) An ideal batch reactor is to be sized for the polymerization of styrene monomer to polystyrene. Pure styrene monomer containing a small amount of the free- radical initiator 2,2'-azobisisobutyronitrile (AIBN) will be charged to the reactor initially, as a liquid. At the end of the polymerization, the reactor will contain a liquid comprised of dissolved polymer, unconverted styrene, and unconverted AIBN. In the following, styrene monomer is denoted as M and AIBN is denoted as I. The AIBN initiator decomposes by a first-order reaction to form two free radicals I 2R The rate constant for this reaction (based on AIBN) is k. Each free radical starts one polymer chain. K3KB PB "IB "MIBE (1 + KMPM + KBPB) k4KMPMKB PB (1 + KMPM + KB PB) where k = 0.23 mol/gcat-h, k = 0.10 mol/gcat-h, k3 = 1.10 mol/gcat-h, k4 2.78 mol/gcat-h, KB = 0.0243 kPa, and KM = 0.0137kPa 1.3 What is the composition of the stream leaving the reactor?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the composition of the stream leaving the reactor lets break down the process into several steps Step 1 Understand the Reactions 1 Initiation ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


