Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Problem 8 A 0.50% C carbon steel is heated to 1000 C and equilibrated; then it is slowly cooled to 600 C, where it
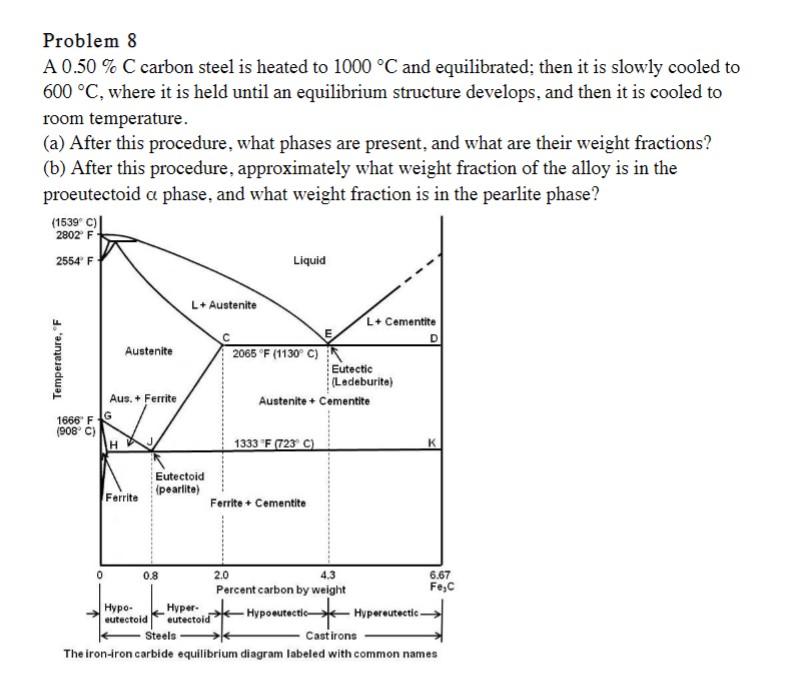
Problem 8 A 0.50% C carbon steel is heated to 1000 C and equilibrated; then it is slowly cooled to 600 C, where it is held until an equilibrium structure develops, and then it is cooled to room temperature. (a) After this procedure, what phases are present, and what are their weight fractions? (b) After this procedure, approximately what weight fraction of the alloy is in the proeutectoid a phase, and what weight fraction is in the pearlite phase? (1539 C) 2802 F 2554 F Liquid Temperature, "F 1666 F (908 C) L+ Austenite L+ Cementite Austenite 2065 F (1130 C) Eutectic (Ledeburite) Aus. + Ferrite Austenite + Cementite H 1333 F (723 C) Eutectoid (pearlite) Ferrite Ferrite + Cementite 0 0.8 - eutectoid Hyper- eutectoid Steels K 2.0 4.3 Percent carbon by weight 6.67 Fe,C -Hypoeutectic- Hypereutectic. Castirons The iron-iron carbide equilibrium diagram labeled with common names
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


