

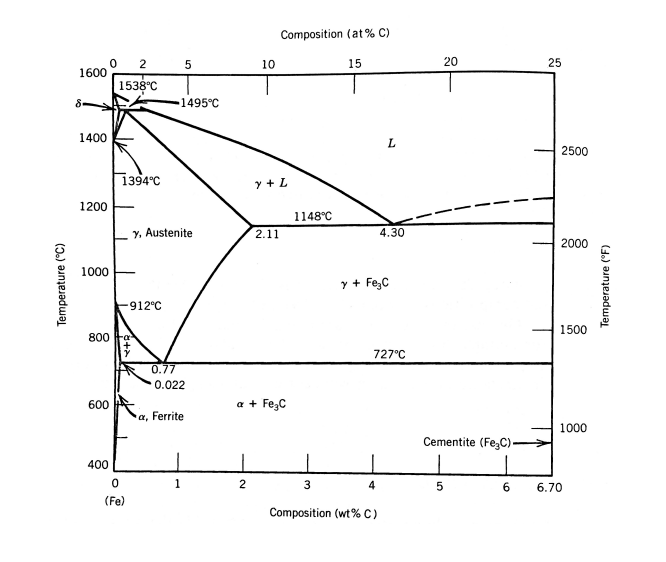
Problem 8 For most dispersion-hardened alloys, usually it is reasonable to assume that all particles are the same size. However, this is not always accurate. Sometimes there is a range of particle sizes and it is convenient to consider that there are two particle sizes, one with a large diameter and one with a small diameter. Swords produced several hundred years ago in the Middle East were often made with hypereutectoid steel, with a composition of about Fe-1.8 wt. % C, with other assorted minor impurities that we will neglect for this problem. The processing of these swords has been reconstructed as the following: 1. Low carbon steel was annealed with charcoal at about 1200 C, until the carbon level reached about 1.8 wt. %. 2. The steel was then cooled very slowly. This allowed a network of coarse proeutectoid cementite to form at the boundaries of the austenite grains. 3. The steel was then reheated to about 750 C and forged into shape (recall that the eutectoid temperature is 727 C). During this forging, the proeutectoid cementite grains were broken up into fairly large spherical particles of approximately 1 um in diameter and approximately 10 m apart. 4. The steel was then quenched to room temperature. This quenching converted the remaining austenite into martensite. 5. Finally, the steel was tempered at temperatures between 400 C and 600 C. During this tem- pering, the martensite was converted to tempered martensite consisting of very fine grained ferrite with a dispersion of very fine cementite particles. The final grain size of the ferrite is about 1 micron (10-6 m, 1 um). The carbon concentration remaining in the ferrite after tempering is estimated to be about 0.05 wt. %. This is slightly above equilibrium because the precipitation of the fine cementite was not performed to completion during the tempering The final product of the process is a very hard and tough sword blade. From hardness measure- ments, the yield strength is estimated to be 1800 MPa. This yield strength results from several strengthening mechanisms, which in a first analysis can be assumed to provide additive contribu- tions to the total yield strength value: Olattice + solution + grain-size + Odislocations + Opartides Rewriting for easier mathematical notation: oy = OL+os+og+op top Let's analyze this material and the strength contributions of different microconstituents in the ma- terial. First, let's provide some preliminary information: The Fe-C phase diagram is provided following this problem. Lattice friction (Peierls stress) of Fe at room temperature: OL = 60 MPa. Solid solution strengthening due 0.05 wt. % C in Fe: os = 200 MPa. Assume the strengthening due to large and small particles is due to a particle looping mechanism, where strength can be calculated according to the following: 4Gb OP L Here, G is the shear modulus of Fe, b is the Burgers vector, and L is the average particle separation, which was provided earlier. G = 7.8 x 104 MPa, b = 2.46 x 10-10 m. m Assume the grain size strengthening and dislocation strengthening can be calculated as follows, where d is grain diameter, ky = 21 MPa-mm!/2 is a grain size strengthening constant, and p* is the dislocation density: 0g = kyd- op = Gbp Analysis to perform: (a) The proeutectoid cementite comprises the large particles in the steel microstructure. Use the lever rule to determine the mass fraction of large particles. (b) What is the contribution of the large particles to the yield stress? (c) What is the contribution of ferrite grain size strengthening to the yield stress? (d) If the dislocation density is estimated to be p* = 1013 m-%, what is the contribution of dislo- cation density to the yield stress? (e) What is the volume fraction of the fine cementite particles that precipitate during the temper- ing? Remember to use Co = 0.05 % in order to account for the carbon that remains in solution in the a ferrite. Assume that all phases have the same density. (f) Use the other information you have calculated or which is provided above in order to determine the small particle contribution to the yield strength. Use this stress value to determine the inter- particle spacing L for the small particles. (g) Assume that particle radius r, volume fraction Vf, and interparticle spacing L can be related according to the following: Vp (Tr)/L?. Calculate the average diameter of the small particles. Composition (at % C) 5 10 15 20 25 0 2 1600 1538C 8 1495C 1400 L 2500 1394C Y+L 1200 1148C 7. Austenite 2.11 4.30 2000 1000 Temperature (C) Y + Fe,C Temperature (F) 912C 1500 800 ta 727C 0.77 0.022 600 a + Fezc a, Ferrite 1000 1 Cementite (Fe,c) 400 1 2 3 4 5 6 6.70 (Fe) Composition (wt% C)









