Problem Set 2: Please fill out the whole table Use the online software to build the 3D structure of the molecule shown below. Save
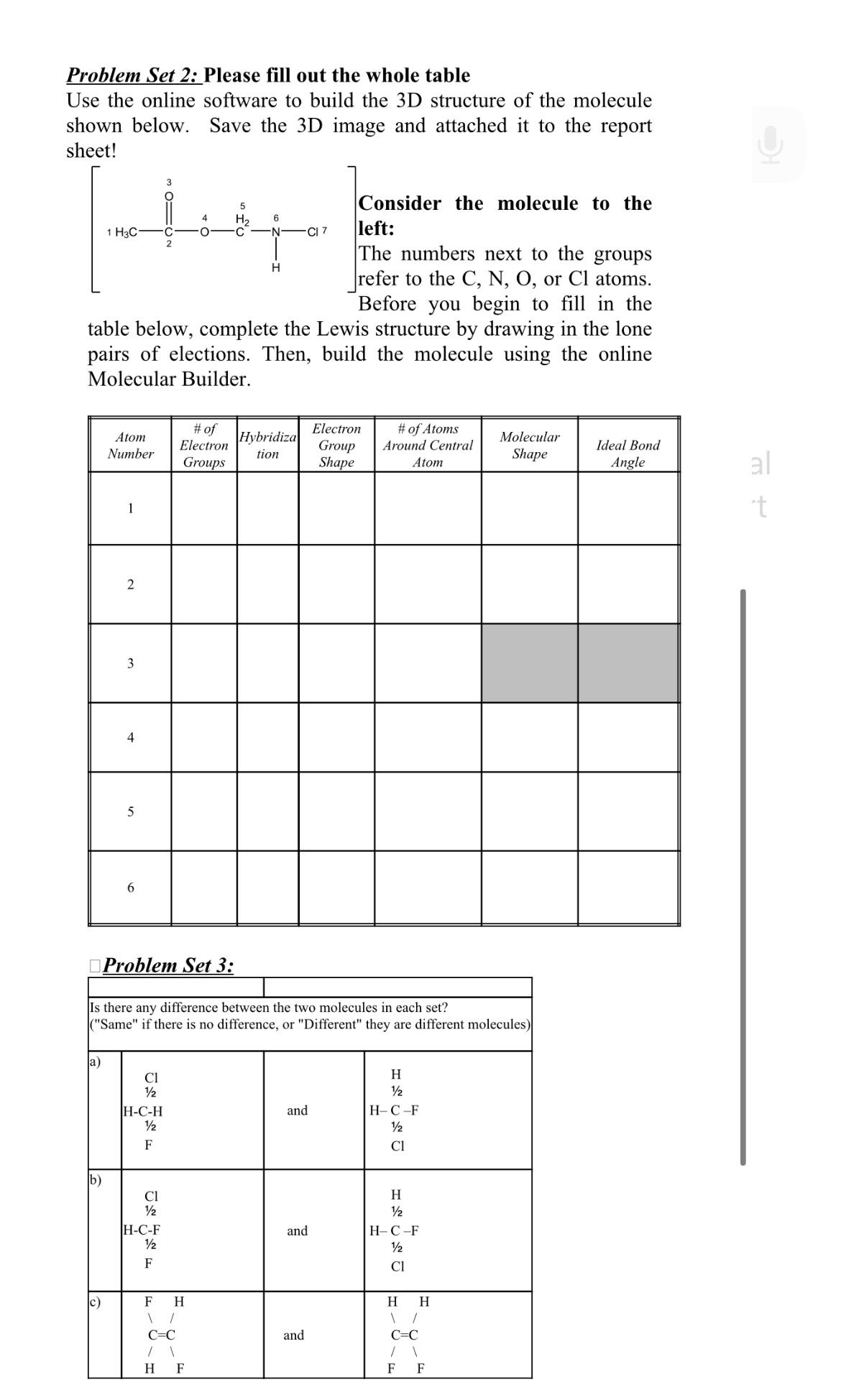
Problem Set 2: Please fill out the whole table Use the online software to build the 3D structure of the molecule shown below. Save the 3D image and attached it to the report sheet! 1 H3C 2 H CI 7 Consider the molecule to the left: The numbers next to the groups refer to the C, N, O, or Cl atoms. Before you begin to fill in the table below, complete the Lewis structure by drawing in the lone pairs of elections. Then, build the molecule using the online Molecular Builder. Atom Number # of Electron Groups Hybridiza tion Electron Group Shape # of Atoms Around Central Atom Molecular Shape Ideal Bond Angle al 1 + t 2 3 4 5 6 Problem Set 3: Is there any difference between the two molecules in each set? ("Same" if there is no difference, or "Different" they are different molecules) a) 1 Cl H 1/2 1/2 H-C-H and H-C-F 1/2 1/2 F Cl b) 1 Cl H 1/2 H-C-F and H-C-F 1/2 F Cl C) F H H H \ 1 C=C and C=C / \ H F F F
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The image presents two chemistry problems The first is to fill out a table that includes the molecular geometry and other attributes for the given mol...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


