Answered step by step
Verified Expert Solution
Question
1 Approved Answer
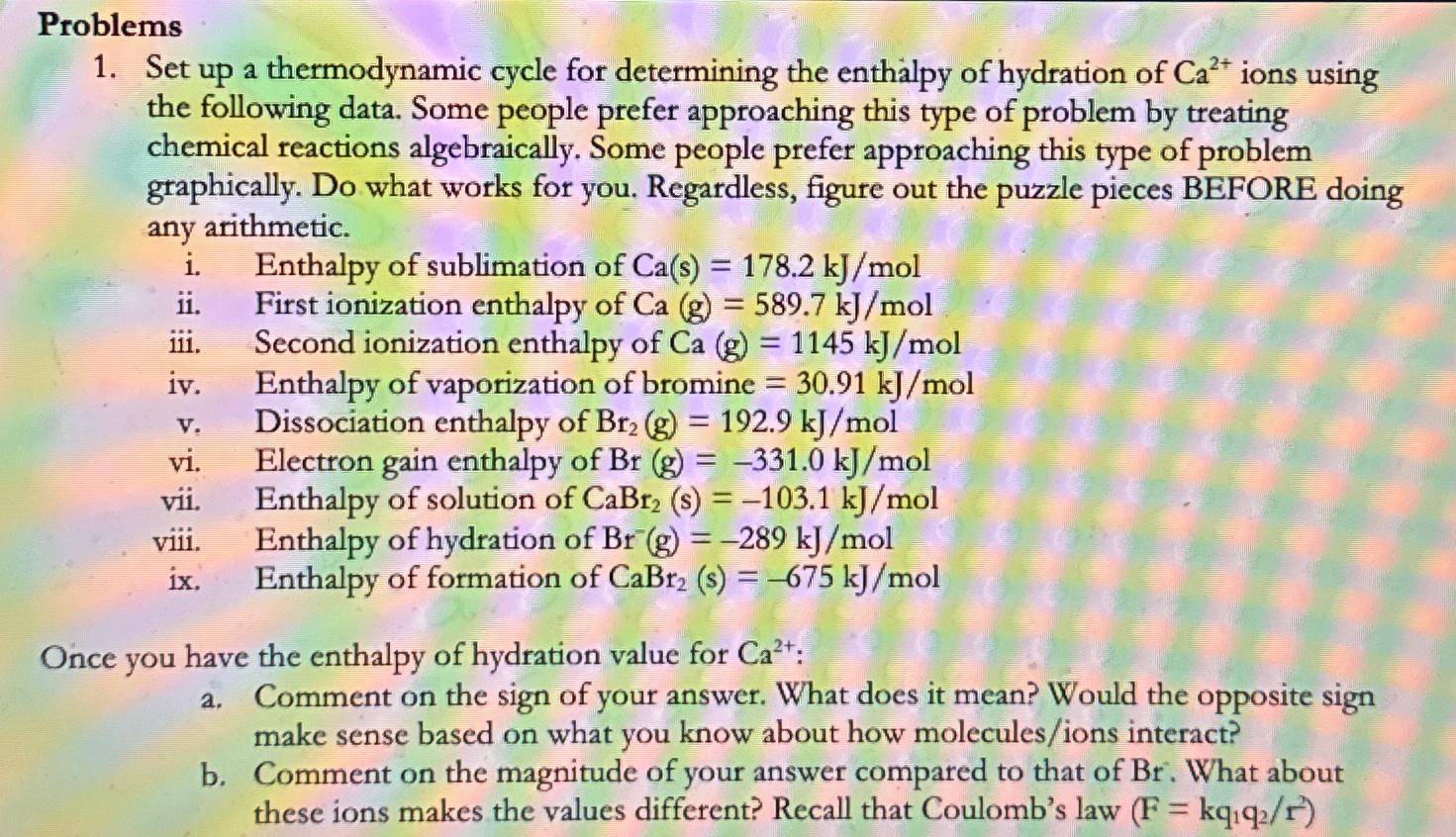
Problems Set up a thermodynamic cycle for determining the enthalpy of hydration of C a 2 + ions using the following data. Some people prefer
Problems
Set up a thermodynamic cycle for determining the enthalpy of hydration of ions using the following data. Some people prefer approaching this type of problem by treating chemical reactions algebraically. Some people prefer approaching this type of problem graphically. Do what works for you. Regardless, figure out the puzzle pieces BEFORE doing any arithmetic.
i Enthalpy of sublimation of
ii First ionization enthalpy of
iii. Second ionization enthalpy of
iv Enthalpy of vaporization of bromine
v Dissociation enthalpy of
vi Electron gain enthalpy of
vii. Enthalpy of solution of
viii. Enthalpy of hydration of
ix Enthalpy of formation of
Once you have the enthalpy of hydration value for :
a Comment on the sign of your answer. What does it mean? Would the opposite sign make sense based on what you know about how moleculesions interact?
b Comment on the magnitude of your answer compared to that of What about these ions makes the values different? Recall that Coulomb's law
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


