Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PROCEDURE 1. Thoroughly clean the pycnometer and stopper with a surfactant cleaning fluid, rinse well with distilled water. Finally rinse with acetone and dry. 2.
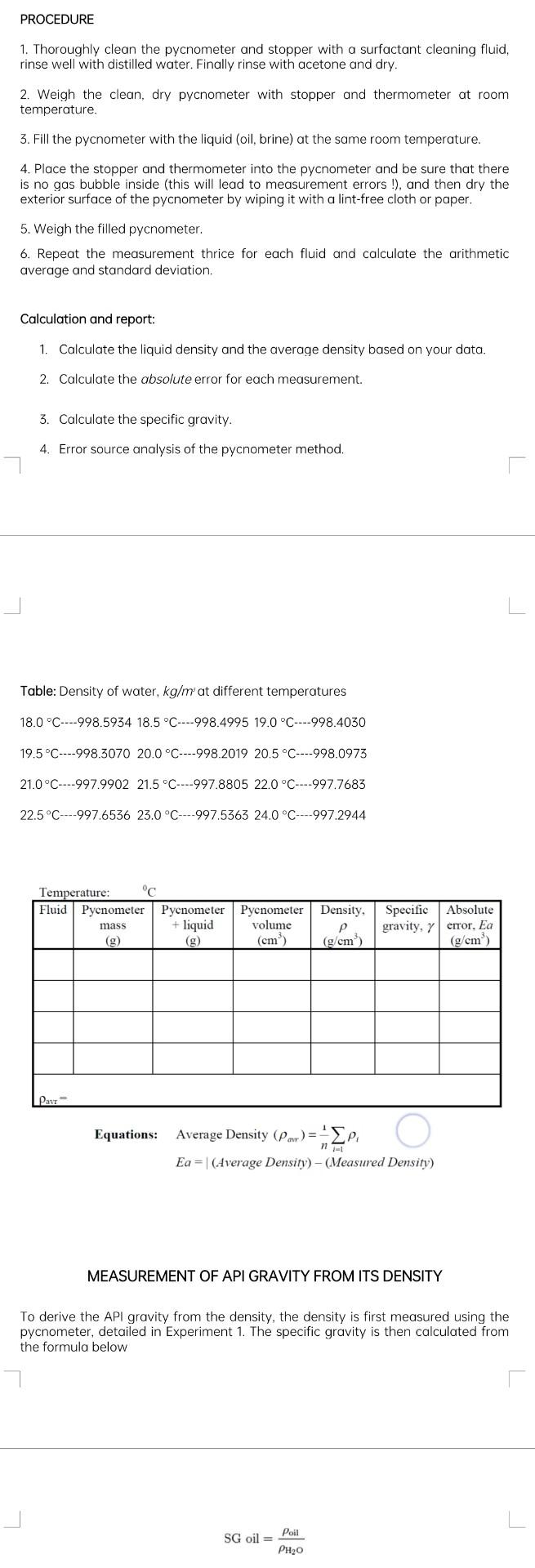
PROCEDURE 1. Thoroughly clean the pycnometer and stopper with a surfactant cleaning fluid, rinse well with distilled water. Finally rinse with acetone and dry. 2. Weigh the clean, dry pycnometer with stopper and thermometer at room temperature. 3. Fill the pycnometer with the liquid (oil, brine) at the same room temperature. 4. Place the stopper and thermometer into the pycnometer and be sure that there is no gas bubble inside (this will lead to measurement errors !), and then dry the exterior surface of the pycnometer by wiping it with a lint-free cloth or paper. 5. Weigh the filled pycnometer. 6. Repeat the measurement thrice for each fluid and calculate the arithmetic average and standard deviation. Calculation and report: 1. Calculate the liquid density and the average density based on your data. 2. Calculate the absolute error for each measurement. 3. Calculate the specific gravity. 4. Error source analysis of the pycnometer method. Table: Density of water, kg/ms at different temperatures 18.0C998.593418.5C998.499519.0C998.4030 19.5C998.307020.0C998.201920.5C998.0973 21.0C997.990221.5C997.880522.0C997.7683 22.5C997.653623.0C997.536324.0C997.2944 Equations: Average Density (arr)=n1i=1i Ea= (Average Density) ( Measured Density) MEASUREMENT OF API GRAVITY FROM ITS DENSITY To derive the API gravity from the density, the density is first measured using the pycnometer, detailed in Experiment 1. The specific gravity is then calculated from the formula below SG oil =H2Ooil PROCEDURE 1. Thoroughly clean the pycnometer and stopper with a surfactant cleaning fluid, rinse well with distilled water. Finally rinse with acetone and dry. 2. Weigh the clean, dry pycnometer with stopper and thermometer at room temperature. 3. Fill the pycnometer with the liquid (oil, brine) at the same room temperature. 4. Place the stopper and thermometer into the pycnometer and be sure that there is no gas bubble inside (this will lead to measurement errors !), and then dry the exterior surface of the pycnometer by wiping it with a lint-free cloth or paper. 5. Weigh the filled pycnometer. 6. Repeat the measurement thrice for each fluid and calculate the arithmetic average and standard deviation. Calculation and report: 1. Calculate the liquid density and the average density based on your data. 2. Calculate the absolute error for each measurement. 3. Calculate the specific gravity. 4. Error source analysis of the pycnometer method. Table: Density of water, kg/ms at different temperatures 18.0C998.593418.5C998.499519.0C998.4030 19.5C998.307020.0C998.201920.5C998.0973 21.0C997.990221.5C997.880522.0C997.7683 22.5C997.653623.0C997.536324.0C997.2944 Equations: Average Density (arr)=n1i=1i Ea= (Average Density) ( Measured Density) MEASUREMENT OF API GRAVITY FROM ITS DENSITY To derive the API gravity from the density, the density is first measured using the pycnometer, detailed in Experiment 1. The specific gravity is then calculated from the formula below SG oil =H2Ooil
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


