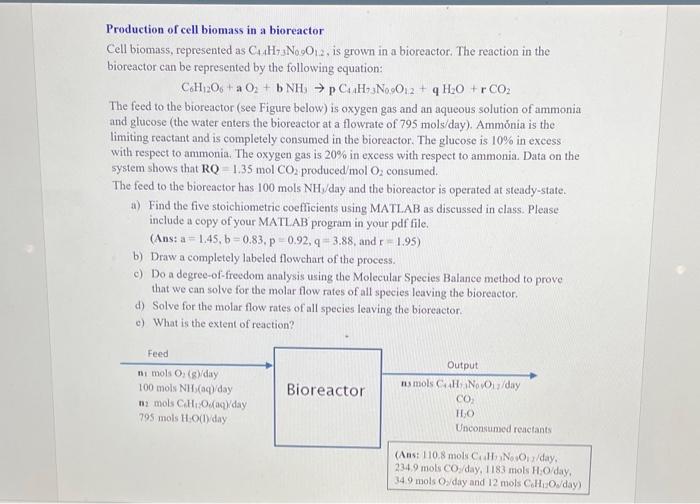
Production of cell biomass in a bioreactor Cell biomass, represented as C4H+3N0012, is grown in a bioreactor. The reaction in the bioreactor can be represented by the following equation: C.H206 +a 02 + b NH, p C H: No 0:2 + H2O + r CO2 The feed to the bioreactor (see Figure below) is oxygen gas and an aqueous solution of ammonia and glucose (the water enters the bioreactor at a flowrate of 795 mols/day). Ammnia is the limiting reactant and is completely consumed in the bioreactor. The glucose is 10% in excess with respect to ammonia. The oxygen gas is 20% in excess with respect to ammonia. Data on the system shows that RO 1.35 mol CO2 produced mol O consumed. The feed to the bioreactor has 100 mols NH/day and the bioreactor is operated at steady-state. a) Find the five stoichiometric coefficients using MATLAB as discussed in class. Please include a copy of your MATLAB program in your pdf file. (Ans: a = 1.45, b=0.83,p 0.92,4- 3.88, and r 1.95) b) Draw a completely labeled flowchart of the process. c) Do a degree of freedom analysis using the Molecular Species Balance method to prove that we can solve for the molar flow rates of all species leaving the bioreactor. d) Solve for the molar flow rates of all species leaving the bioreactor. c) What is the extent of reaction? Feed mi mols O. (syday 100 mols NH3(aq)day 12 mois CHO (1) day 795 mols Holyday Bioreactor Output nu mols CN. O/day CO HO Unconsumed reactants (Anst 110.8 mols Call Nox/day, 234.9 mois Cos/day, 1183 mols O/day, 34.9 mols Oday and 12 mois CHO/day) Production of cell biomass in a bioreactor Cell biomass, represented as C4H+3N0012, is grown in a bioreactor. The reaction in the bioreactor can be represented by the following equation: C.H206 +a 02 + b NH, p C H: No 0:2 + H2O + r CO2 The feed to the bioreactor (see Figure below) is oxygen gas and an aqueous solution of ammonia and glucose (the water enters the bioreactor at a flowrate of 795 mols/day). Ammnia is the limiting reactant and is completely consumed in the bioreactor. The glucose is 10% in excess with respect to ammonia. The oxygen gas is 20% in excess with respect to ammonia. Data on the system shows that RO 1.35 mol CO2 produced mol O consumed. The feed to the bioreactor has 100 mols NH/day and the bioreactor is operated at steady-state. a) Find the five stoichiometric coefficients using MATLAB as discussed in class. Please include a copy of your MATLAB program in your pdf file. (Ans: a = 1.45, b=0.83,p 0.92,4- 3.88, and r 1.95) b) Draw a completely labeled flowchart of the process. c) Do a degree of freedom analysis using the Molecular Species Balance method to prove that we can solve for the molar flow rates of all species leaving the bioreactor. d) Solve for the molar flow rates of all species leaving the bioreactor. c) What is the extent of reaction? Feed mi mols O. (syday 100 mols NH3(aq)day 12 mois CHO (1) day 795 mols Holyday Bioreactor Output nu mols CN. O/day CO HO Unconsumed reactants (Anst 110.8 mols Call Nox/day, 234.9 mois Cos/day, 1183 mols O/day, 34.9 mols Oday and 12 mois CHO/day)