Question
Production of formaldehyde (CHO) in a continuous reactor involves the catalytic oxidation of methanol (CHOH): CHOH + 1/2O ---> CHO + HO The reaction
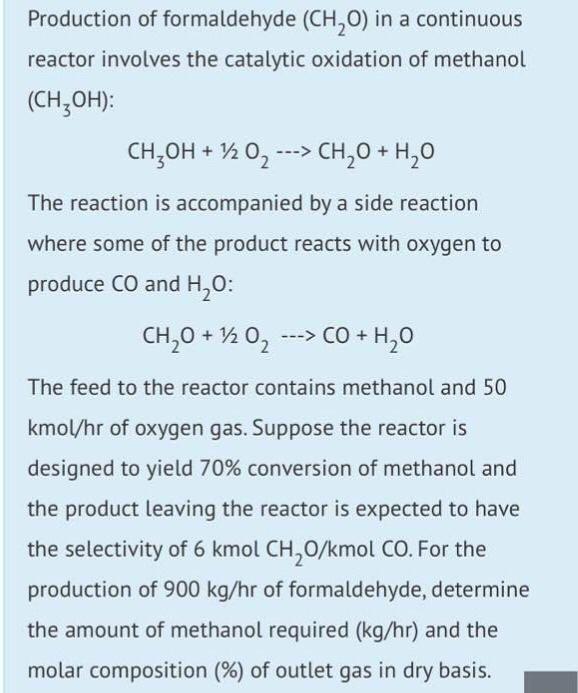
Production of formaldehyde (CHO) in a continuous reactor involves the catalytic oxidation of methanol (CHOH): CHOH + 1/2O ---> CHO + HO The reaction is accompanied by a side reaction where some of the product reacts with oxygen to produce CO and HO: CHO + 1/2O ---> CO + HO The feed to the reactor contains methanol and 50 kmol/hr of oxygen gas. Suppose the reactor is designed to yield 70% conversion of methanol and the product leaving the reactor is expected to have the selectivity of 6 kmol CHO/kmol CO. For the production of 900 kg/hr of formaldehyde, determine the amount of methanol required (kg/hr) and the molar composition (%) of outlet gas in dry basis.
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
1 CHOH 12O CHO HO Bulancing equation 2 moles mole As the reactor Imulas I bemol sohnol n70 2moles 2k...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



