
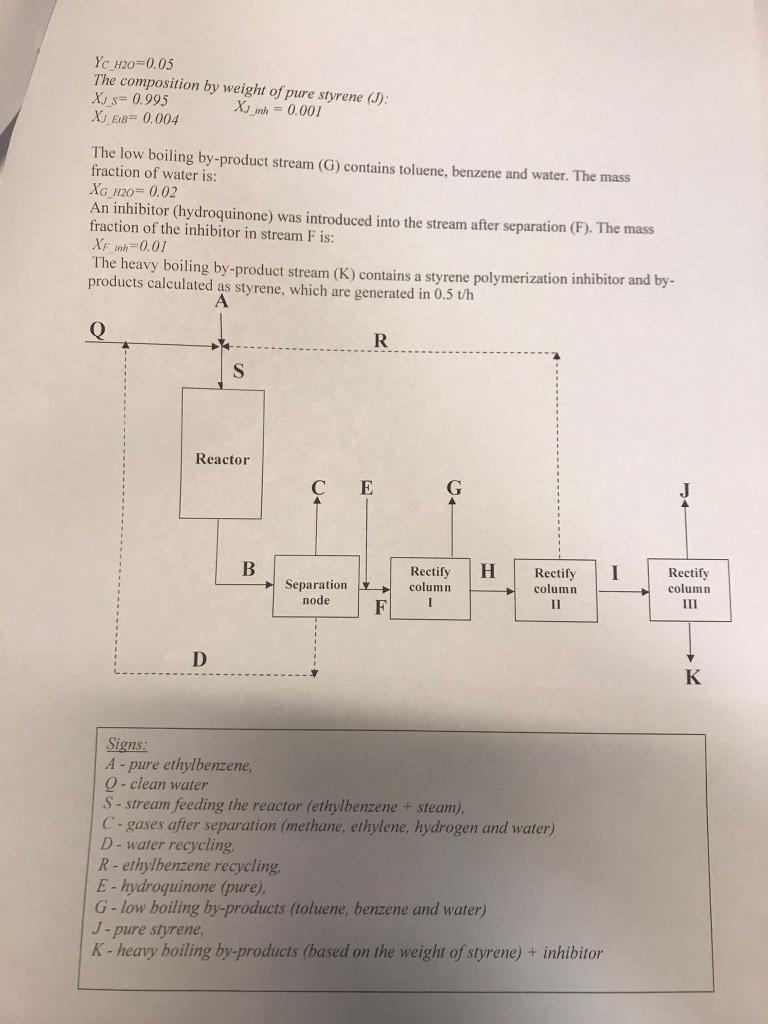
Project Schedule: 29. Description of the processes for obtaining styrene, its use in chemical synthesis and economic analysis of the market (maximum 3 pages). 30. A detailed description of the process of dehydrogenation of ethylbenzene to styrene (in an isothermal reactor), used catalysts and process conditions (maximum 2 pages). 31. Thermodynamic analysis of the dehydrogenation process: - determine the number of independent reactions and propose a stoichiometric model of the process, - calculate thermodynamic equilibrium pressure constants for the reaction of a stoichiometric model, - calculate the equilibrium composition for given process conditions, bypassing side reactions (only for the dehydrogenation reaction of ethylbenzene to styrene in the presence of water vapor). 32. Material balance of the process: - calculate the mass flow of ethylbenzene and steam needed to produce 45t/h stream of purified styrene (page J) with the composition given below. - calculate the masses of all streams and their composition in mole percent, - calculate thermodynamic process efficiency, - make a transparent tabular listing for individual devices and for the entire process. 33. Make drawings: - block diagram for the material balance with a description of individual streams, - technological scheme of the dehydrogenation of ethylbenzene in an isothermal reactor, - Sankey chart for material balance. 34. Waste management. 35. List of source literature. Assumptions: - pure raw materials (water and ethylbenzene) are used, - the gases after separation contain only methane, ethylene, hydrogen and water, - toluene and benzene are distilled off completely on column 1 , - the calculations do not take into account the conversion of hydrocarbon to carbon deposit and the gasification reaction of the deposit with steam. Process conditions: T=600C P=0.24MP KEtB=0.4 - total ethylbenzene conversion in one pass SS=0.92,SB=0.07,ST=0.01 - selectivity of ethylbenzene conversion according to styrene (SS), benzene (SB) itoluene (ST) Molar ratio of raw material at the entrance to the reactor (S) : H2O/EtB=8/1 The composition of gases after separation in volume fractions (C) : YCH2O=0.05 The composition by weight of pure styrene (J) : The low boiling by-product stream (G) contains toluene, benzene and water. The mass fraction of water is: XGH2O=0.02 An inhibitor (hydroquinone) was introduced into the stream after separation (F). The mass fraction of the inhibitor in stream F is: XFinh=0.01 The heavy boiling by-product stream (K) contains a styrene polymerization inhibitor and byproducts calculated as styrene, which are generated in 0.5th Signs: A-pure ethylbenzene, Q - clean water S - stream feeding the reactor (ethylbenzene + steam). C-gases after separation (methane, ethylene, hydrogen and water) D-water recycling. R - ethylbenzene recycling, E- hydroquinone (pure), G-low boiling by-products (toluene, benzene and water) J-pure styrene, K - heavy boiling by-products (based on the weight of styrene) + inhibitor Project Schedule: 29. Description of the processes for obtaining styrene, its use in chemical synthesis and economic analysis of the market (maximum 3 pages). 30. A detailed description of the process of dehydrogenation of ethylbenzene to styrene (in an isothermal reactor), used catalysts and process conditions (maximum 2 pages). 31. Thermodynamic analysis of the dehydrogenation process: - determine the number of independent reactions and propose a stoichiometric model of the process, - calculate thermodynamic equilibrium pressure constants for the reaction of a stoichiometric model, - calculate the equilibrium composition for given process conditions, bypassing side reactions (only for the dehydrogenation reaction of ethylbenzene to styrene in the presence of water vapor). 32. Material balance of the process: - calculate the mass flow of ethylbenzene and steam needed to produce 45t/h stream of purified styrene (page J) with the composition given below. - calculate the masses of all streams and their composition in mole percent, - calculate thermodynamic process efficiency, - make a transparent tabular listing for individual devices and for the entire process. 33. Make drawings: - block diagram for the material balance with a description of individual streams, - technological scheme of the dehydrogenation of ethylbenzene in an isothermal reactor, - Sankey chart for material balance. 34. Waste management. 35. List of source literature. Assumptions: - pure raw materials (water and ethylbenzene) are used, - the gases after separation contain only methane, ethylene, hydrogen and water, - toluene and benzene are distilled off completely on column 1 , - the calculations do not take into account the conversion of hydrocarbon to carbon deposit and the gasification reaction of the deposit with steam. Process conditions: T=600C P=0.24MP KEtB=0.4 - total ethylbenzene conversion in one pass SS=0.92,SB=0.07,ST=0.01 - selectivity of ethylbenzene conversion according to styrene (SS), benzene (SB) itoluene (ST) Molar ratio of raw material at the entrance to the reactor (S) : H2O/EtB=8/1 The composition of gases after separation in volume fractions (C) : YCH2O=0.05 The composition by weight of pure styrene (J) : The low boiling by-product stream (G) contains toluene, benzene and water. The mass fraction of water is: XGH2O=0.02 An inhibitor (hydroquinone) was introduced into the stream after separation (F). The mass fraction of the inhibitor in stream F is: XFinh=0.01 The heavy boiling by-product stream (K) contains a styrene polymerization inhibitor and byproducts calculated as styrene, which are generated in 0.5th Signs: A-pure ethylbenzene, Q - clean water S - stream feeding the reactor (ethylbenzene + steam). C-gases after separation (methane, ethylene, hydrogen and water) D-water recycling. R - ethylbenzene recycling, E- hydroquinone (pure), G-low boiling by-products (toluene, benzene and water) J-pure styrene, K - heavy boiling by-products (based on the weight of styrene) + inhibitor








