Answered step by step
Verified Expert Solution
Question
1 Approved Answer
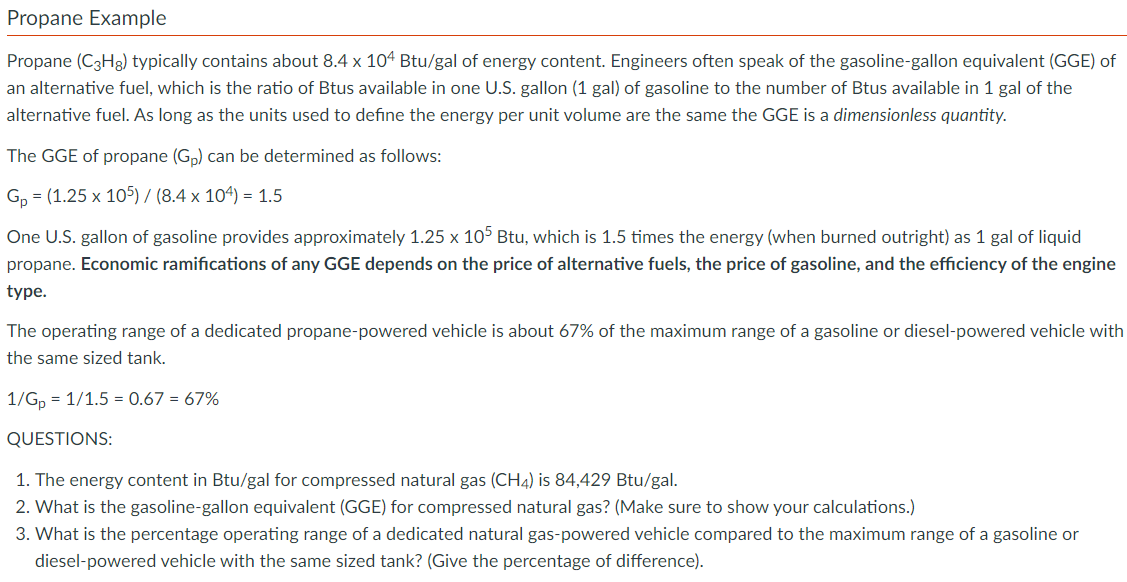
Propane Example Propane ( C 3 H 8 ) typically contains about 8 . 4 1 0 4 B t u ? gal of energy
Propane Example
Propane typically contains about gal of energy content. Engineers often speak of the gasolinegallon equivalent GGE of
an alternative fuel, which is the ratio of Btus available in one US gallon gal of gasoline to the number of Btus available in gal of the
alternative fuel. As long as the units used to define the energy per unit volume are the same the GGE is a dimensionless quantity.
The GGE of propane can be determined as follows:
One US gallon of gasoline provides approximately Btu, which is times the energy when burned outright as gal of liquid
propane. Economic ramifications of any GGE depends on the price of alternative fuels, the price of gasoline, and the efficiency of the engine
type.
The operating range of a dedicated propanepowered vehicle is about of the maximum range of a gasoline or dieselpowered vehicle with
the same sized tank.
QUESTIONS:
The energy content in Btugal for compressed natural gas is
What is the gasolinegallon equivalent GGE for compressed natural gas? Make sure to show your calculations.
What is the percentage operating range of a dedicated natural gaspowered vehicle compared to the maximum range of a gasoline or
dieselpowered vehicle with the same sized tank? Give the percentage of difference
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


