Answered step by step
Verified Expert Solution
Question
1 Approved Answer
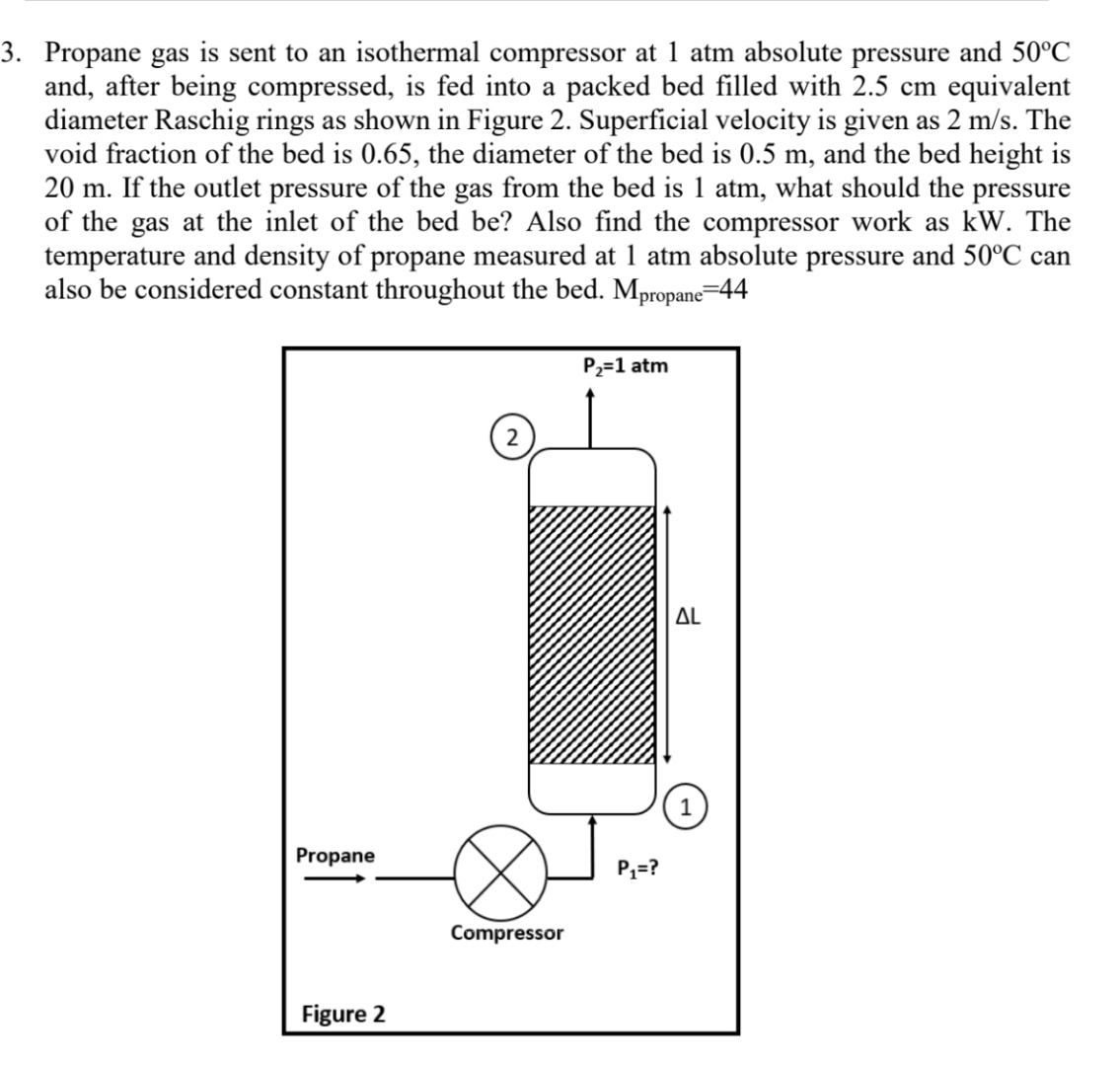
Propane gas is sent to an isothermal compressor at 1 atm absolute pressure and 5 0 C and, after being compressed, is fed into a
Propane gas is sent to an isothermal compressor at atm absolute pressure and and, after being compressed, is fed into a packed bed filled with equivalent diameter Raschig rings as shown in Figure Superficial velocity is given as The void fraction of the bed is the diameter of the bed is and the bed height is If the outlet pressure of the gas from the bed is atm, what should the pressure of the gas at the inlet of the bed be Also find the compressor work as kW The temperature and density of propane measured at atm absolute pressure and can also be considered constant throughout the bed.
Figure
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


