Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave. Please I need the
Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave.
Please I need the algorithm, as it is written in the boldface. Thanks!!
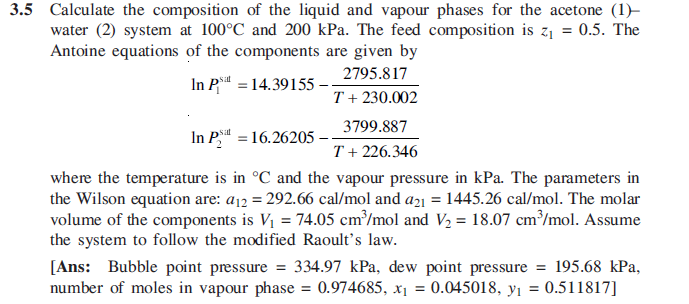
3.5 Calculate the composition of the liquid and vapour phases for the acetone (1) water (2) system at 100C and 200 kPa. The feed composition is zz = 0.5. The Antoine equations of the components are given by In P** = 14.39155 2795.817 T + 230.002 3799.887 In P = 16.26205 T + 226.346 where the temperature is in C and the vapour pressure in kPa. The parameters in the Wilson equation are: 012 = 292.66 cal/mol and a21 = 1445.26 cal/mol. The molar volume of the components is V1 = 74.05 cm/mol and V2 = 18.07 cm/mol. Assume the system to follow the modified Raoult's law. [Ans: Bubble point pressure = 334.97 kPa, dew point pressure = 195.68 kPa, number of moles in vapour phase = 0.974685, x = 0.045018, y = 0.511817] =Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


