Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. A proton exchange membrane fuel cell is fed a fuel gas of H2 from the anode side, while the cathode side is fed
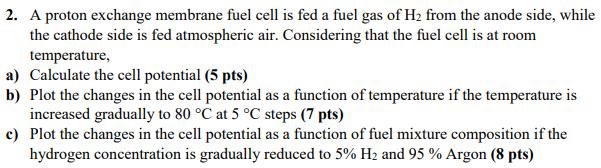
2. A proton exchange membrane fuel cell is fed a fuel gas of H2 from the anode side, while the cathode side is fed atmospheric air. Considering that the fuel cell is at room temperature, a) Calculate the cell potential (5 pts) b) Plot the changes in the cell potential as a function of temperature if the temperature is increased gradually to 80 C at 5 C steps (7 pts) c) Plot the changes in the cell potential as a function of fuel mixture composition if the hydrogen concentration is gradually reduced to 5% H2 and 95 % Argon (8 pts)
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
The chemical energy held in hydrogen fuel is converted into electricity by a hydrogen fuel cell Fuel cells are similar to batteries in many aspects such as those found in cars or portable electrical d...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


