Answered step by step
Verified Expert Solution
Question
1 Approved Answer
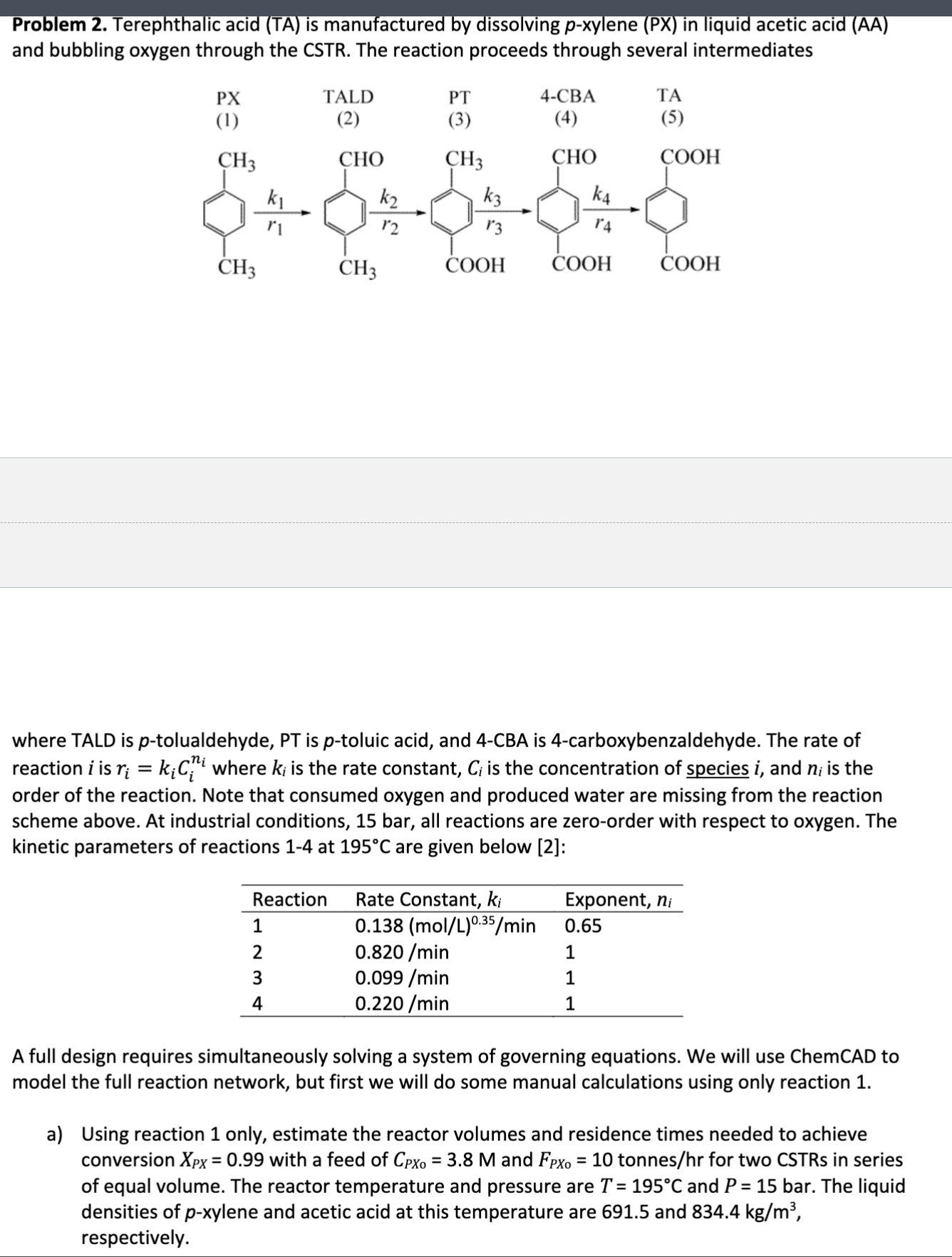
PT 4 - CBA TA where TALD is p - tolualdehyde, PT is p - toluic acid, and 4 - CBA is 4 - carboxybenzaldehyde.
PT
CBA
TA
where TALD is tolualdehyde, PT is toluic acid, and CBA is carboxybenzaldehyde. The rate of
reaction is where is the rate constant, is the concentration of species and is the
order of the reaction. Note that consumed oxygen and produced water are missing from the reaction
scheme above. At industrial conditions, bar, all reactions are zeroorder with respect to oxygen. The
kinetic parameters of reactions at are given below :
A full design requires simultaneously solving a system of governing equations. We will use ChemCAD to
model the full reaction network, but first we will do some manual calculations using only reaction
a Using reaction only, estimate the reactor volumes and residence times needed to achieve
conversion with a feed of and tonneshr for two CSTRs in series
of equal volume. The reactor temperature and pressure are and bar. The liquid
densities of xylene and acetic acid at this temperature are and
respectively.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


