Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the value of Attrxn for the following - ZBH, g) B H16 (9) (9) reaction? (A) + 130 kJ/mol (B) +10 kJ/mol
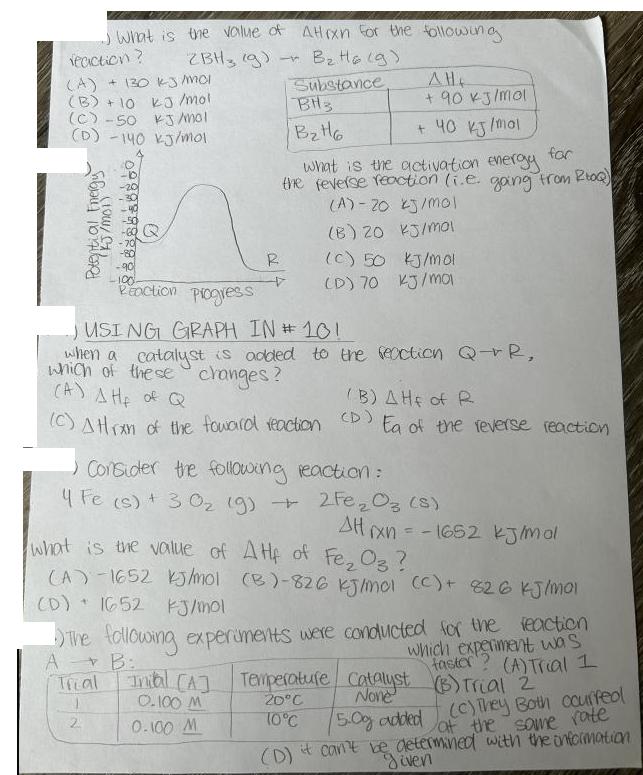
What is the value of Attrxn for the following - ZBH, g) B H16 (9) (9) reaction? (A) + 130 kJ/mol (B) +10 kJ/mol (C)-50 kJ/mol Substance (D) -140 kJ/mol Potential Energy (100/1) -b BH3 BHo AH + 90 kJ/mol + 40 kJ/mol what is the activation energy for the feverse reaction (i.e. gang from RoQ) (A) 20 kJ/mol (B) 20 kJ/mol R (c) 50 kJ/mol (D) 70 kJ/mol Reaction progress -) USING GRAPH IN #101 when a catalyst is added to the reaction Q-R, which of these changes? (A) AH of Q (B) AHE of R (c) Atran of the foward reaction (D) Ea of the reverse reaction Consider the following reaction: Fe (s) + 302 (9) + 2Fe2O3 (8) AHxn=-1652 kJ/mol what is the value of AHp of Fe2O3 ? (A)-1652 kJ/mol (B)-826 kJ/mol (C) + 826 kJ/mol (D) 1652 kJ/mol A The following experiments were conducted for the reaction Trial B: 20C Inial ([A] Temperature Catalyst None 0.100 M 2 0.100 M 10C which experiment was faster? (A) Trial I (B) Trial 2 (5.0g added (c) They Both courfeol at the same rate (D) it can't be determined with the information given
Step by Step Solution
★★★★★
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
The activation energy for the reverse reaction is calculated as the negative of the activation ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


