Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pure water is used to dilute 152 lbm of a sulfuric acid stream that has an initial mass fraction of H2SO4 equal to 0.85
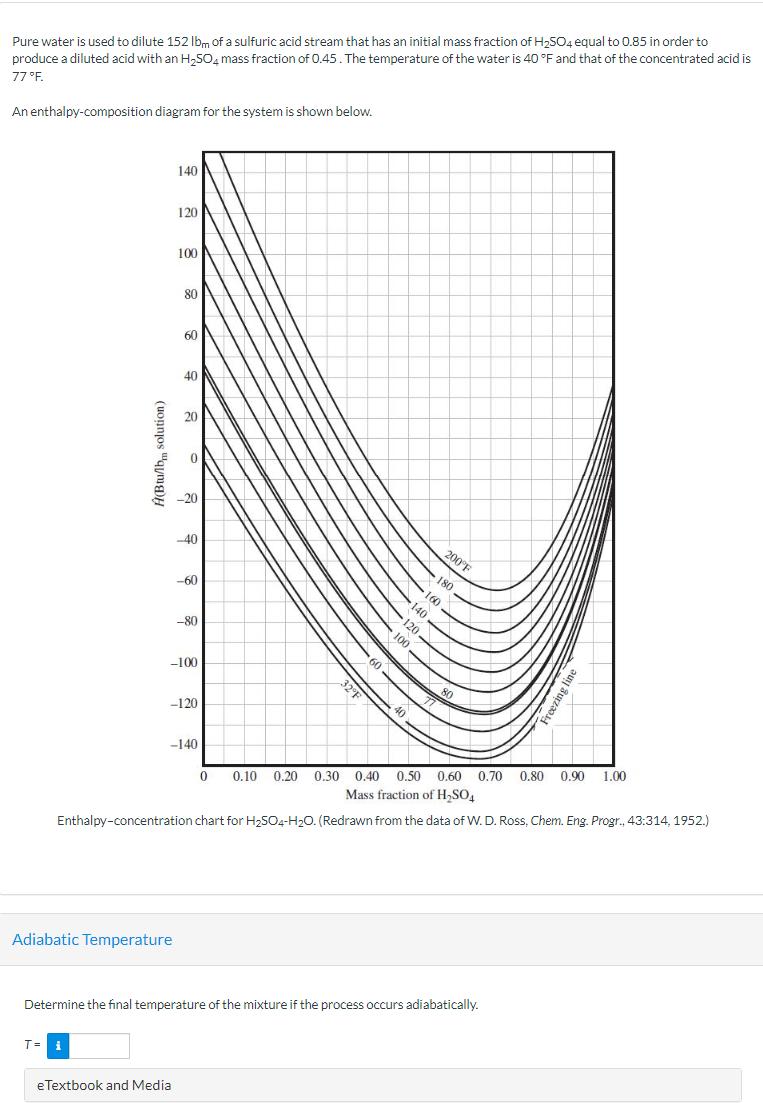
Pure water is used to dilute 152 lbm of a sulfuric acid stream that has an initial mass fraction of H2SO4 equal to 0.85 in order to produce a diluted acid with an H504 mass fraction of 0.45. The temperature of the water is 40 F and that of the concentrated acid is 77F. An enthalpy-composition diagram for the system is shown below. (Btu/lb solution) 140 120 100 80 60 40 20 -20 -40 -60 -80 -100 -120 -140 60 40 32F 200F 80 Freezing line 0 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 Mass fraction of HSO4 Enthalpy-concentration chart for H2504-H2O. (Redrawn from the data of W. D. Ross, Chem. Eng. Progr., 43:314, 1952.) Adiabatic Temperature Determine the final temperature of the mixture if the process occurs adiabatically. T= i eTextbook and Media
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Determining the Final Temperature of the Mixture Heres how to determine the final temperature of the mixture in this adiabatic mixing process Calculate the total mass of the mixture Mass of concentrat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


