Answered step by step
Verified Expert Solution
Question
1 Approved Answer
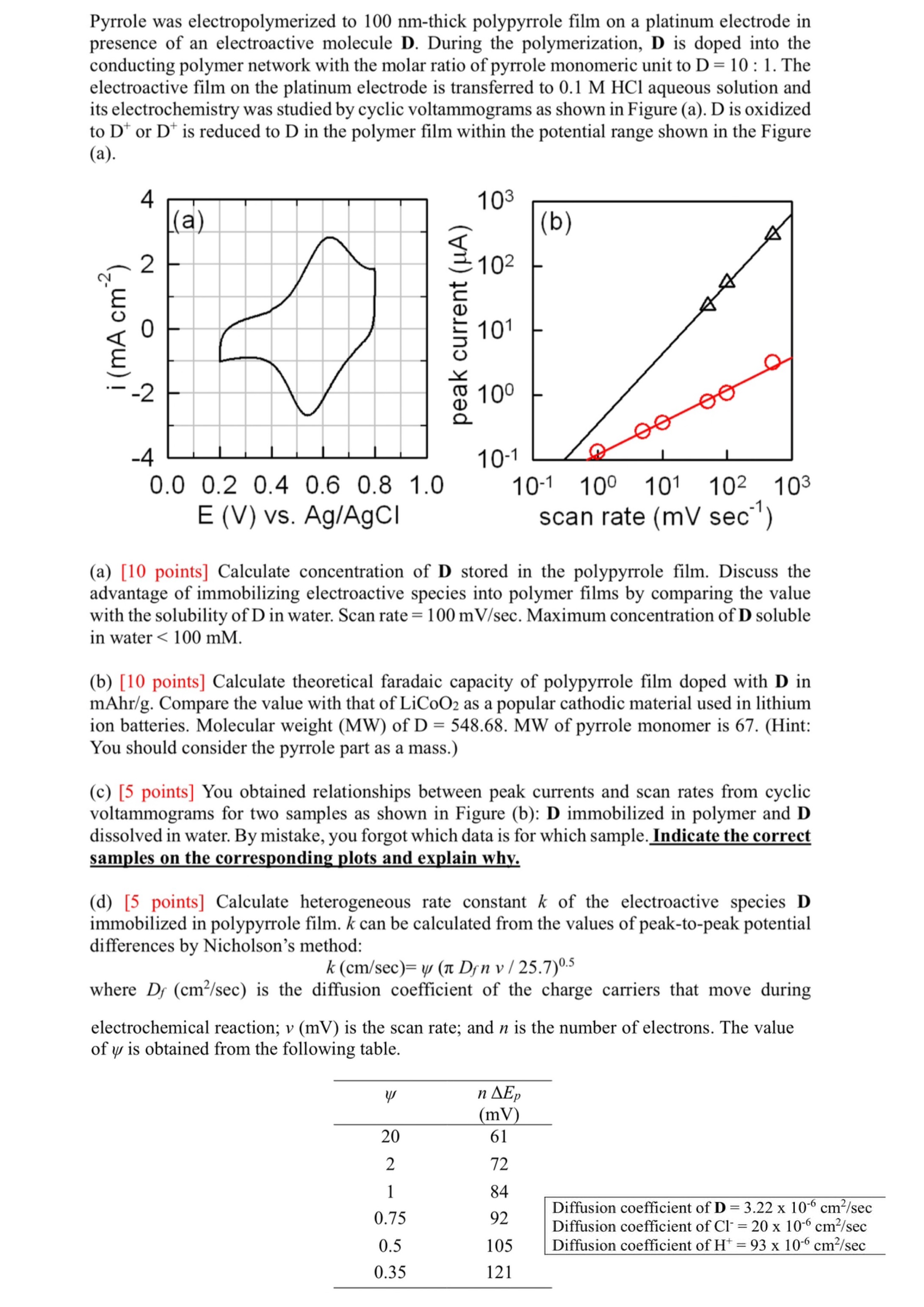
Pyrrole was electropolymerized to 1 0 0 n m - thick polypyrrole film on a platinum electrode in presence of an electroactive molecule D .
Pyrrole was electropolymerized to thick polypyrrole film on a platinum electrode in
presence of an electroactive molecule D During the polymerization, D is doped into the
conducting polymer network with the molar ratio of pyrrole monomeric unit to : The
electroactive film on the platinum electrode is transferred to aqueous solution and
its electrochemistry was studied by cyclic voltammograms as shown in Figure a D is oxidized
to or is reduced to in the polymer film within the potential range shown in the Figure
a
a points Calculate concentration of stored in the polypyrrole film. Discuss the
advantage of immobilizing electroactive species into polymer films by comparing the value
with the solubility of D in water. Scan rate Maximum concentration of D soluble
in water
b points Calculate theoretical faradaic capacity of polypyrrole film doped with in
mAh Compare the value with that of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


