Answered step by step
Verified Expert Solution
Question
1 Approved Answer
p(z) = p(0) e (z/H) 1) (20 PTS) Using the equation above, show that the total mass m of the species in the atmosphere
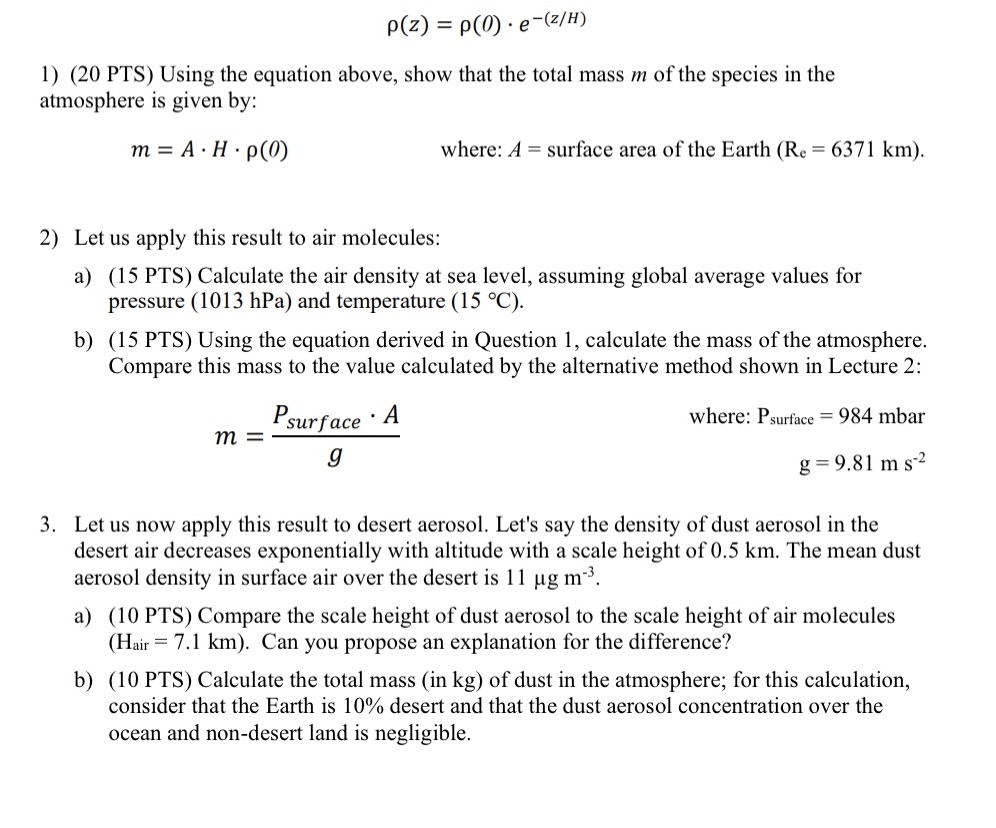
p(z) = p(0) e (z/H) 1) (20 PTS) Using the equation above, show that the total mass m of the species in the atmosphere is given by: m = A H. p(0) where: A surface area of the Earth (Re = 6371 km). 2) Let us apply this result to air molecules: a) (15 PTS) Calculate the air density at sea level, assuming global average values for pressure (1013 hPa) and temperature (15 C). b) (15 PTS) Using the equation derived in Question 1, calculate the mass of the atmosphere. Compare this mass to the value calculated by the alternative method shown in Lecture 2: Psurface . A m = g where: Psurface = 984 mbar g=9.81 ms-2 3. Let us now apply this result to desert aerosol. Let's say the density of dust aerosol in the desert air decreases exponentially with altitude with a scale height of 0.5 km. The mean dust aerosol density in surface air over the desert is 11 g m. a) (10 PTS) Compare the scale height of dust aerosol to the scale height of air molecules (Hair 7.1 km). Can you propose an explanation for the difference? b) (10 PTS) Calculate the total mass (in kg) of dust in the atmosphere; for this calculation, consider that the Earth is 10% desert and that the dust aerosol concentration over the ocean and non-desert land is negligible.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


