Answered step by step
Verified Expert Solution
Question
1 Approved Answer
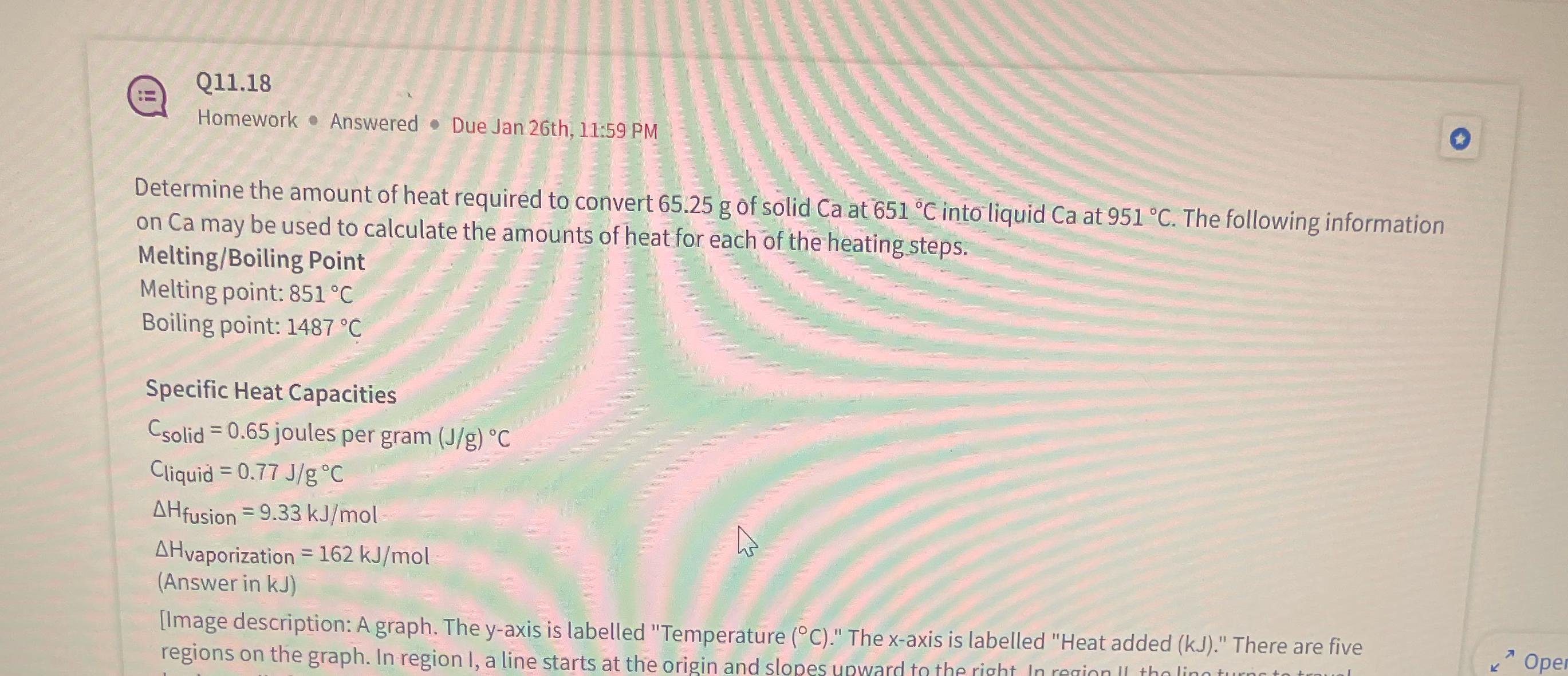
Q 1 1 . 1 8 Homework * Answered - Due Jan 2 6 th , 1 1 : 5 9 PM Determine the amount
Q
Homework Answered Due Jan th: PM
Determine the amount of heat required to convert of solid at into liquid at The following information on Ca may be used to calculate the amounts of heat for each of the heating steps.
MeltingBoiling Point
Melting point:
Boiling point:
Specific Heat Capacities
joules per gram
Cliquid
Answer in
Image description: A graph. The yaxis is labelled "Temperature The axis is labelled "Heat added There are five regions on the graph. In region I, a line starts at the origin and slopes upward to the right
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


