Answered step by step
Verified Expert Solution
Question
1 Approved Answer
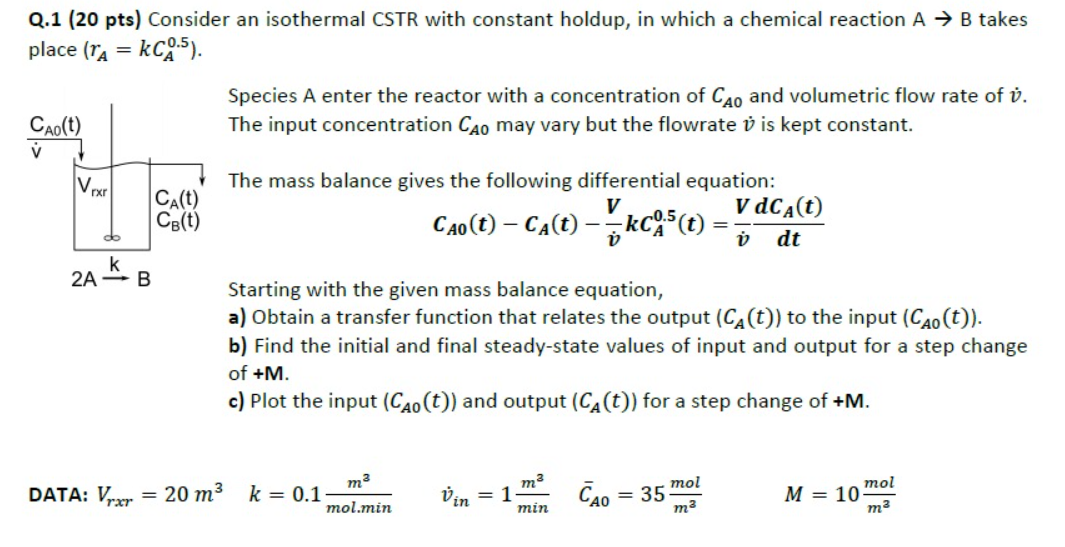
Q . 1 ( 2 0 pts ) Consider an isothermal CSTR with constant holdup, in which a chemical reaction A B takes place (
Q pts Consider an isothermal CSTR with constant holdup, in which a chemical reaction A B takes
place
Species A enter the reactor with a concentration of and volumetric flow rate of
The input concentration may vary but the flowrate is kept constant.
The mass balance gives the following differential equation:
Starting with the given mass balance equation,
a Obtain a transfer function that relates the output to the input
b Find the initial and final steadystate values of input and output for a step change
of
c Plot the input and output for a step change of
DATA:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


