Answered step by step
Verified Expert Solution
Question
1 Approved Answer
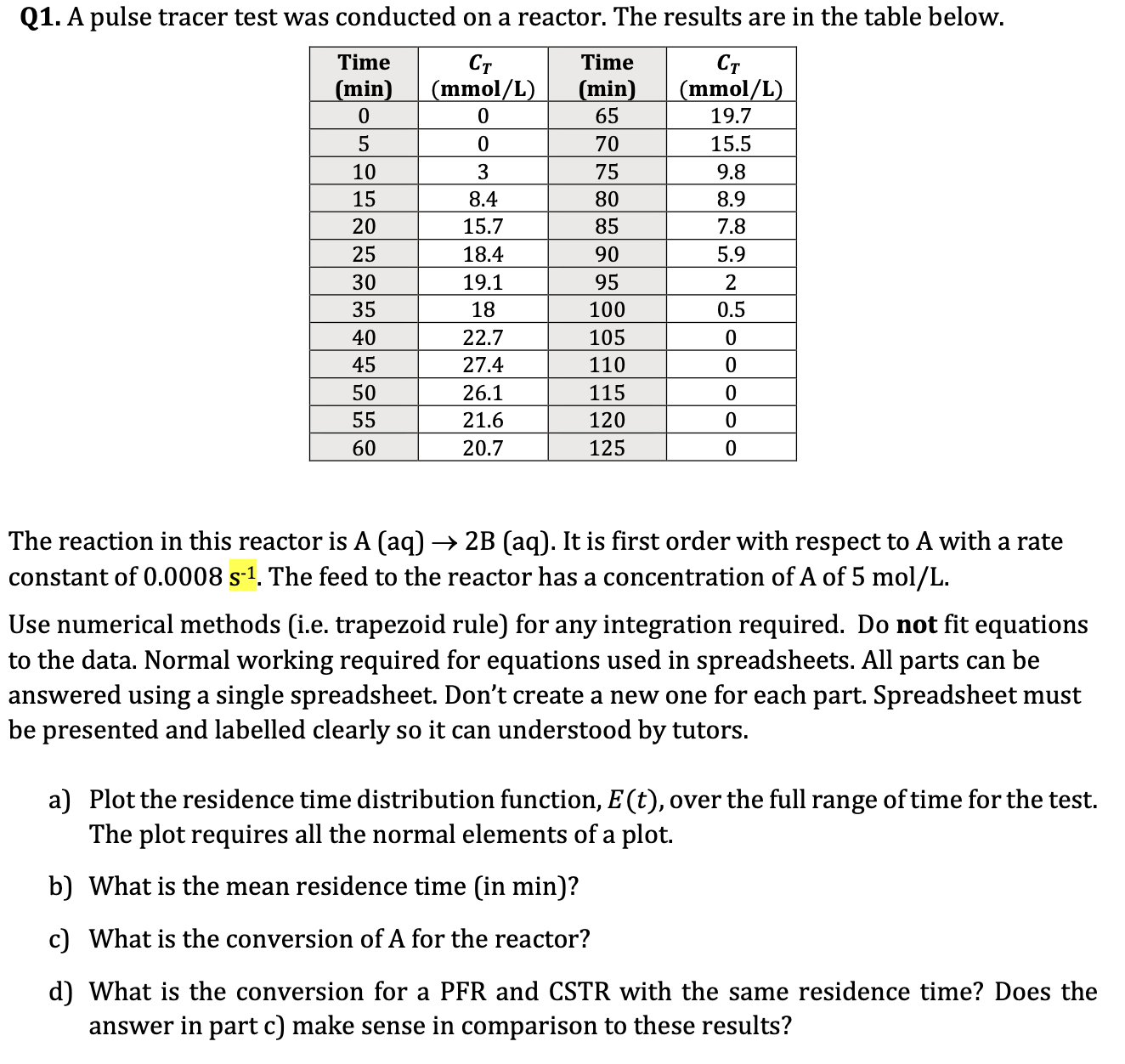
Q 1 . A pulse tracer test was conducted on a reactor. The results are in the table below. The reaction in this reactor is
Q A pulse tracer test was conducted on a reactor. The results are in the table below.
The reaction in this reactor is It is first order with respect to A with a rate
constant of The feed to the reactor has a concentration of A of
Use numerical methods ie trapezoid rule for any integration required. Do not fit equations
to the data. Normal working required for equations used in spreadsheets. All parts can be
answered using a single spreadsheet. Don't create a new one for each part. Spreadsheet must
be presented and labelled clearly so it can understood by tutors.
a Plot the residence time distribution function, over the full range of time for the test.
The plot requires all the normal elements of a plot.
b What is the mean residence time in min
c What is the conversion of A for the reactor?
d What is the conversion for a PFR and CSTR with the same residence time? Does the
answer in part c make sense in comparison to these results?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


