Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q 1: An aqueous solution of substance A contains 70% by mass of substance A. 100 kg of water was added to dilute the solution.
Q 1: An aqueous solution of substance A contains 70% by mass of substance A. 100 kg of water was added to dilute the solution. Calculate the volume of the resulting solution (mg) and the mass ratio (3, 3,X) for each of substance A and water (w) m = 200 kg m3 = XA,1 = 0.7 XA3= Xw.10.3 Xw,3 = m2 = 100 kg of w Q2: Depending on the following reaction, which represents the oxidation of ammonia 4NO + 6H20 4NH3 + 502 If 50 kg of ammonia and 100kg of oxygen are fed to the reactor, determine the limiting reactant, the percentage by which the other reactant increases, the extent of the reaction (g) and the mass of NO produced (kg) if the reaction proceeds to 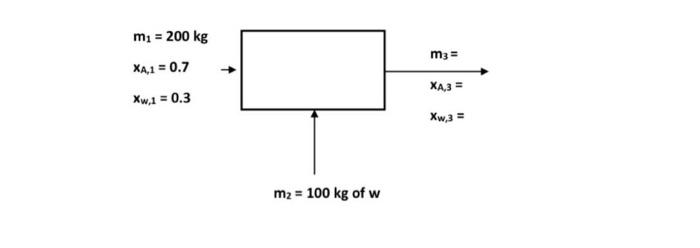

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


