Answered step by step
Verified Expert Solution
Question
1 Approved Answer
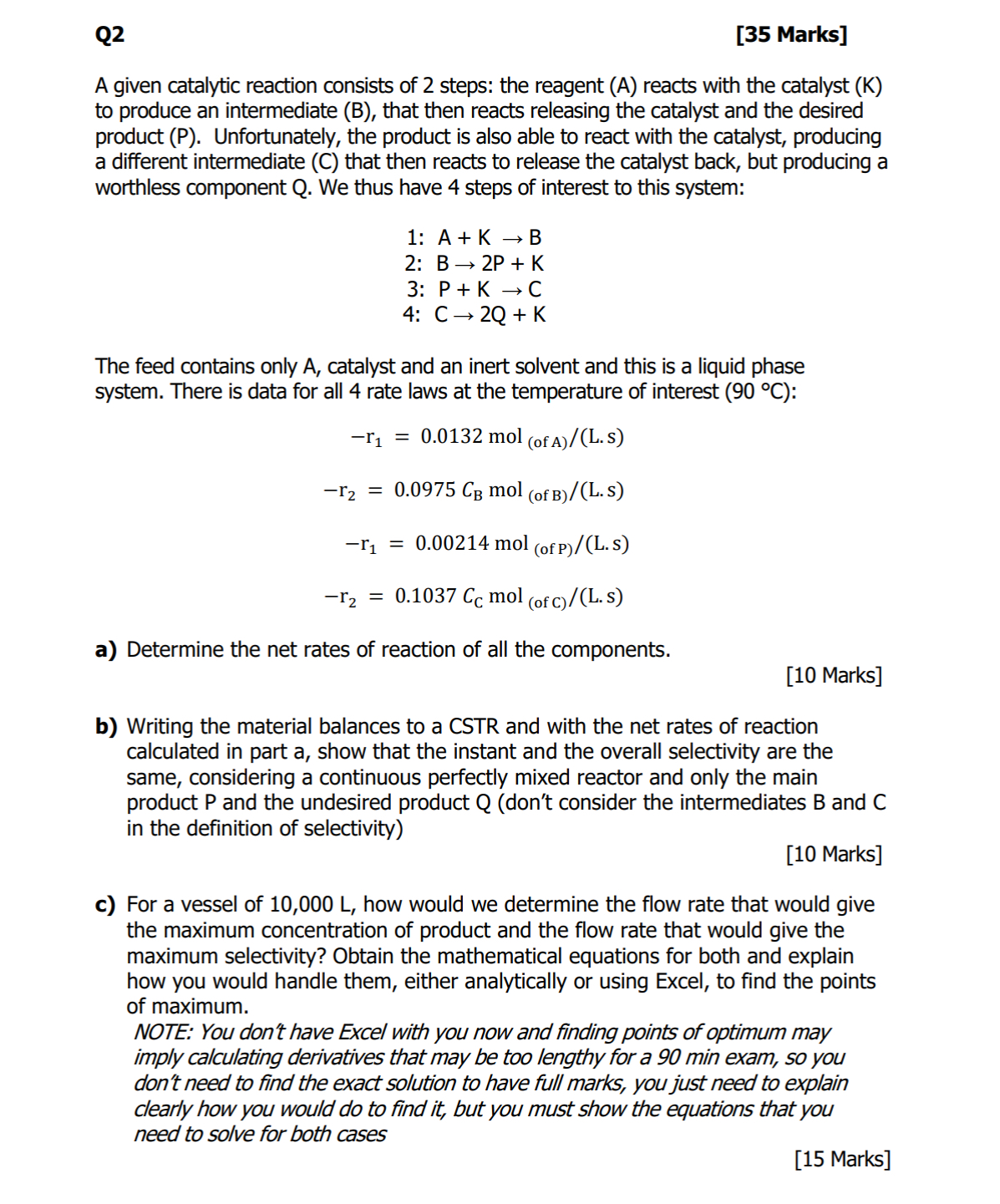
Q 2 [ 3 5 Marks ] A given catalytic reaction consists of 2 steps: the reagent ( A ) reacts with the catalyst (
Q
Marks
A given catalytic reaction consists of steps: the reagent reacts with the catalyst to produce an intermediate B that then reacts releasing the catalyst and the desired product Unfortunately, the product is also able to react with the catalyst, producing a different intermediate C that then reacts to release the catalyst back, but producing a worthless component Q We thus have steps of interest to this system:
:
:
:
:
The feed contains only catalyst and an inert solvent and this is a liquid phase system. There is data for all rate laws at the temperature of interest :
a Determine the net rates of reaction of all the components.
Marks
b Writing the material balances to a CSTR and with the net rates of reaction calculated in part a show that the instant and the overall selectivity are the same, considering a continuous perfectly mixed reactor and only the main product and the undesired product dont consider the intermediates B and C in the definition of selectivity
Marks
c For a vessel of how would we determine the flow rate that would give the maximum concentration of product and the flow rate that would give the maximum selectivity? Obtain the mathematical equations for both and explain how you would handle them, either analytically or using Excel, to find the points of maximum.
NOTE: You don't have Excel with you now and finding points of optimum may imply calculating derivatives that may be too lengthy for a min exam, so you don't need to find the exact solution to have full marks, you just need to explain clearly how you would do to find it but you must show the equations that you need to solve for both cases
Marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


