Answered step by step
Verified Expert Solution
Question
1 Approved Answer
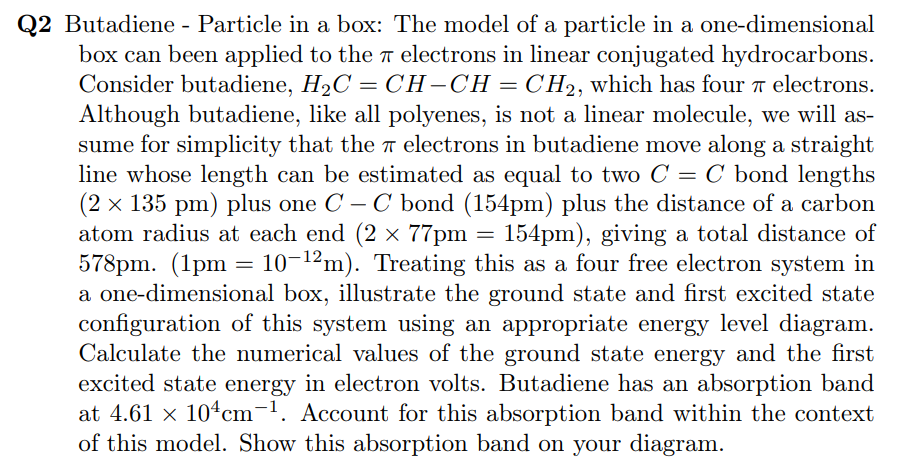
Q 2 Butadiene - Particle in a box: The model of a particle in a one - dimensional box can been applied to the electrons
Q Butadiene Particle in a box: The model of a particle in a onedimensional
box can been applied to the electrons in linear conjugated hydrocarbons.
Consider butadiene, which has four electrons.
Although butadiene, like all polyenes, is not a linear molecule, we will as
sume for simplicity that the electrons in butadiene move along a straight
line whose length can be estimated as equal to two bond lengths
plus one bond plus the distance of a carbon
atom radius at each end giving a total distance of
Treating this as a four free electron system in
a onedimensional box, illustrate the ground state and first excited state
configuration of this system using an appropriate energy level diagram.
Calculate the numerical values of the ground state energy and the first
excited state energy in electron volts. Butadiene has an absorption band
at Account for this absorption band within the context
of this model. Show this absorption band on your diagram.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


