Answered step by step
Verified Expert Solution
Question
1 Approved Answer
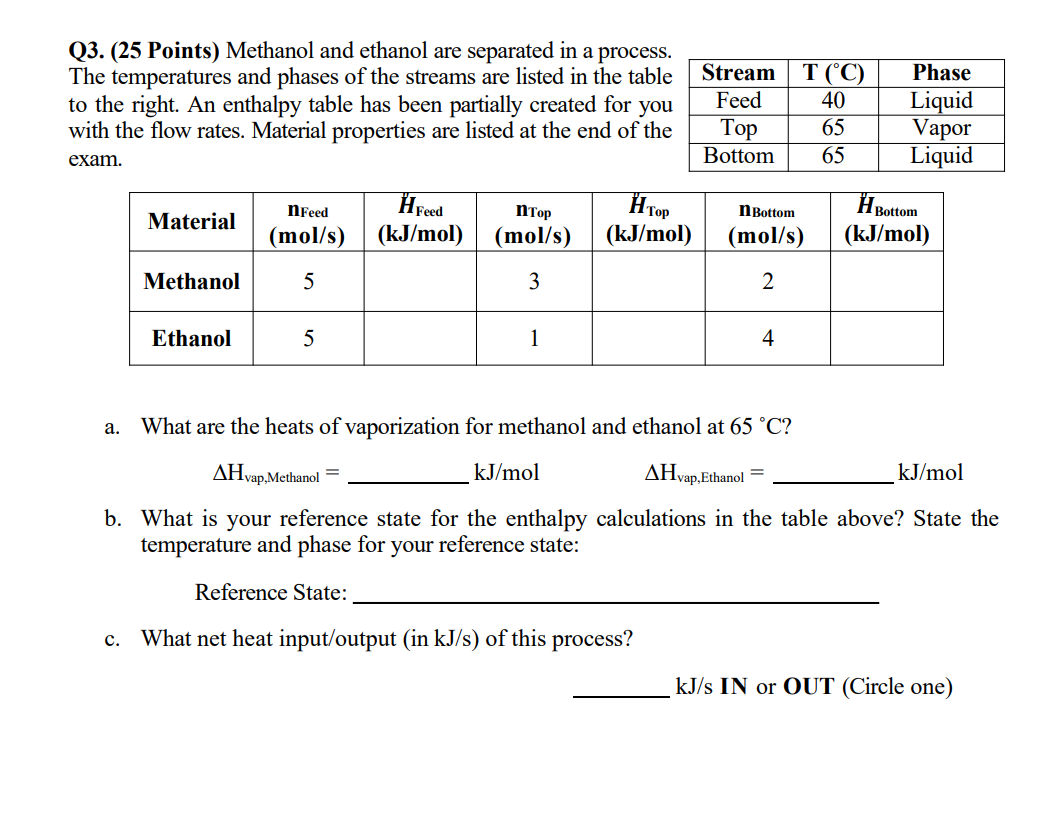
Q 3 . ( 2 5 Points ) Methanol and ethanol are separated in a process. The temperatures and phases of the streams are listed
Q Points Methanol and ethanol are separated in a process.
The temperatures and phases of the streams are listed in the table
to the right. An enthalpy table has been partially created for you
with the flow rates. Material properties are listed at the end of the
exam.
a What are the heats of vaporization for methanol and ethanol at
b What is your reference state for the enthalpy calculations in the table above? State the
temperature and phase for your reference state:
Reference State:
c What net heat inputoutput in of this process?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


