Answered step by step
Verified Expert Solution
Question
1 Approved Answer
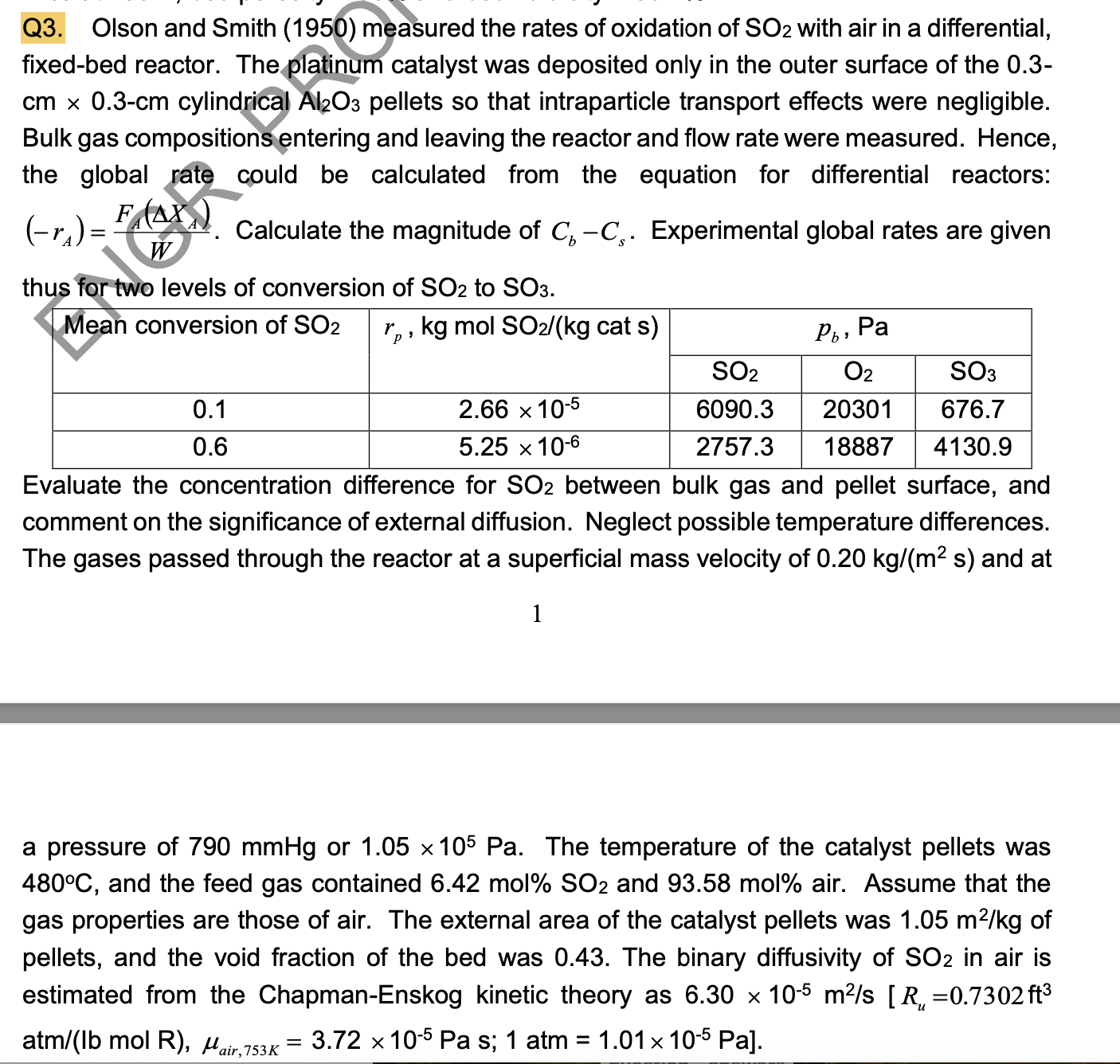
Q 3 . Olson and Smith ( 1 9 5 0 ) measured the rates of oxidation of S O 2 with air in a
Q Olson and Smith measured the rates of oxidation of with air in a differential, fixedbed reactor. The platinum catalyst was deposited only in the outer surface of the cylindrical pellets so that intraparticle transport effects were negligible. Bulk gas compositions entering and leaving the reactor and flow rate were measured. Hence, the global rate could be calculated from the equation for differential reactors: Calculate the magnitude of Experimental global rates are given thus for two levels of conversion of to
tableMean conversion of kgmolS
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


