Answered step by step
Verified Expert Solution
Question
1 Approved Answer
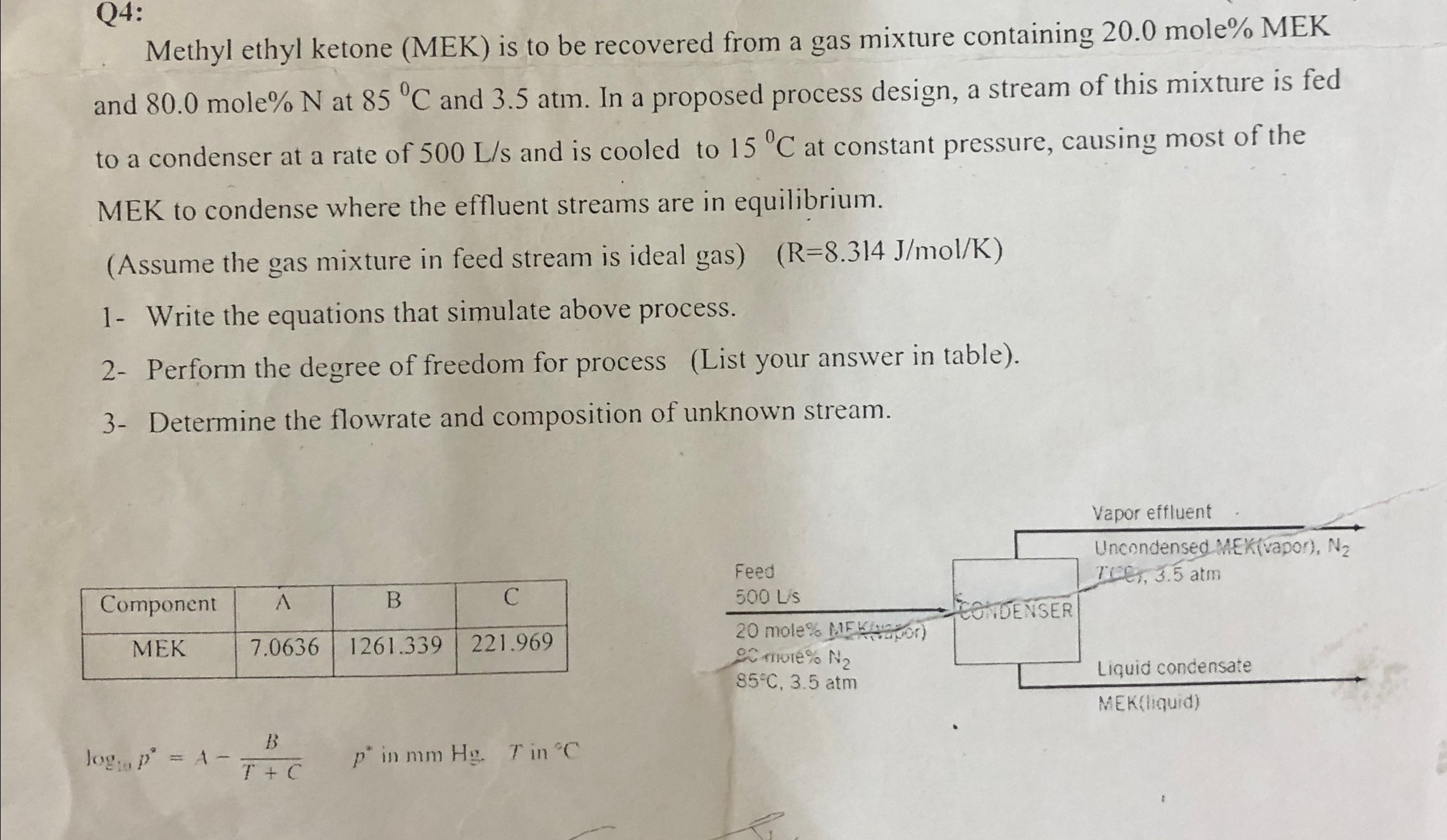
Q 4 : Methyl ethyl ketone ( MEK ) is to be recovered from a gas mixture containing 2 0 . 0 mole % MEK
Q:
Methyl ethyl ketone MEK is to be recovered from a gas mixture containing mole MEK and mole at and atm. In a proposed process design, a stream of this mixture is fed to a condenser at a rate of and is cooled to at constant pressure, causing most of the MEK to condense where the effluent streams are in equilibrium.
Assume the gas mixture in feed stream is ideal gas
Write the equations that simulate above process.
Perform the degree of freedom for process List your answer in table
Determine the flowrate and composition of unknown stream.
tableComponentABCMEK
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


