Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q1) For two Case Studies the following operations were performed: For the production of MgSO47H2O crystals, a solution of 20,000lb/h with 0.300 weight fraction MgSO4

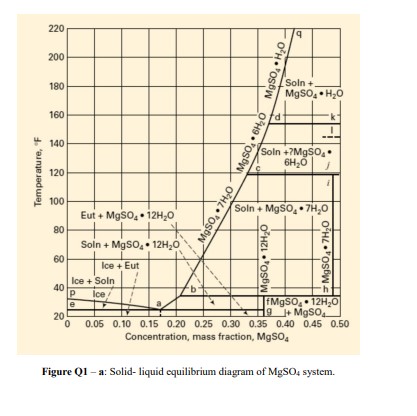
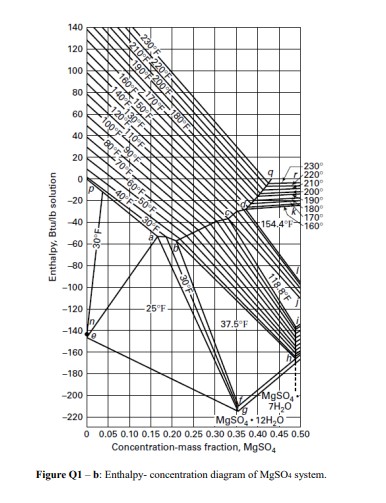 Q1) For two Case Studies the following operations were performed: For the production of MgSO47H2O crystals, a solution of 20,000lb/h with 0.300 weight fraction MgSO4 was sent to a vacuum crystallizer at 160F and 20 psia. The solution was first evaporated until a saturated solution was obtained at temperature 160F. a) determine the weight fraction MgSO4 in the saturated solution at this temperature and draw on the Figure 1. b) determine the amount of saturated solution obtained and water evaporated. In the Case Study 1: This saturated solution was sent to a cooling crystalizer at atmospheric pressure so that magma was formed containing saturated mother liquor and crystals. The crystals produced were 6000lb/h of MgSO47H2O. c) determine the weight percent MgSO4 of the crystals. d) determine the amount of saturated mother liquor and its content of weight percent MgSO4, showing on the Figure Q1-a. e) determine the temperature the magma should be cooled until reaching the 6000lb/h crystals formed. In the Case Study 2: If we use vacuum crystallizer at 70F instead of a cooling crystallizer entering as saturated foed at 160F : f) determine the weight percent of MgSO4 in the mother liquor obtained, g) calculate the amount of mother liquor formed (for crystal amount 6000lb/h ). h) calculate the vapor amount produced. i) calculate the Btu/h of heat addition, using the enthalpy-concentration diagram (Figure Q1-b) and steam table. j) Compare the results of the two cases studied and discuss about the compounds produced in terms of their amounts and purities. Hint: For the calculations neglect boiling point rise in the evaporator. Figure Q1 - a: Solid- liquid equilibrium diagram of MgSO4 system. Concentration-mass fraction, MgSO4 Figure Q1 - b: Enthalpy- concentration diagram of MgSO4 system
Q1) For two Case Studies the following operations were performed: For the production of MgSO47H2O crystals, a solution of 20,000lb/h with 0.300 weight fraction MgSO4 was sent to a vacuum crystallizer at 160F and 20 psia. The solution was first evaporated until a saturated solution was obtained at temperature 160F. a) determine the weight fraction MgSO4 in the saturated solution at this temperature and draw on the Figure 1. b) determine the amount of saturated solution obtained and water evaporated. In the Case Study 1: This saturated solution was sent to a cooling crystalizer at atmospheric pressure so that magma was formed containing saturated mother liquor and crystals. The crystals produced were 6000lb/h of MgSO47H2O. c) determine the weight percent MgSO4 of the crystals. d) determine the amount of saturated mother liquor and its content of weight percent MgSO4, showing on the Figure Q1-a. e) determine the temperature the magma should be cooled until reaching the 6000lb/h crystals formed. In the Case Study 2: If we use vacuum crystallizer at 70F instead of a cooling crystallizer entering as saturated foed at 160F : f) determine the weight percent of MgSO4 in the mother liquor obtained, g) calculate the amount of mother liquor formed (for crystal amount 6000lb/h ). h) calculate the vapor amount produced. i) calculate the Btu/h of heat addition, using the enthalpy-concentration diagram (Figure Q1-b) and steam table. j) Compare the results of the two cases studied and discuss about the compounds produced in terms of their amounts and purities. Hint: For the calculations neglect boiling point rise in the evaporator. Figure Q1 - a: Solid- liquid equilibrium diagram of MgSO4 system. Concentration-mass fraction, MgSO4 Figure Q1 - b: Enthalpy- concentration diagram of MgSO4 system Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


