Question
Q1) Writea balancedchemicalequationforthereactionbetweenH2SO4andNaOH. Q2) ThepHscaleisameasureoftheacidityofasolution,itisbasedonthe[H+]concentrationofthesolution.GiventhiswhydoesthepHincreasewhenthe [H+]concentrationgoesdown? Q3) WhichismoreacidiclemonjuicewhichhasapHof2.2ororangejuicethat hasapHof3.6? Q4) YouneedtomeasurethepHofasolutionprecisely.YouhavepHpaperandapHmeteravailable.ItwilltakelongertousethepHmeter.WhichmethodofmeasuringpHshouldyouuseandwhy? Q5) In the exercise, Bronsted-Lowry Acids and Bases, it was shown that after on
Q1) Writea balancedchemicalequationforthereactionbetweenH2SO4andNaOH.
Q2) ThepHscaleisameasureoftheacidityofasolution,itisbasedonthe[H+]concentrationofthesolution.GiventhiswhydoesthepHincreasewhenthe [H+]concentrationgoesdown?
Q3) WhichismoreacidiclemonjuicewhichhasapHof2.2ororangejuicethat hasapHof3.6?
Q4) YouneedtomeasurethepHofasolutionprecisely.YouhavepHpaperandapHmeteravailable.ItwilltakelongertousethepHmeter.WhichmethodofmeasuringpHshouldyouuseandwhy?
Q5) In the exercise, Bronsted-Lowry Acids and Bases, it was shown that after on acid has given up its proton, it is capable of getting back that proton and acting as a base, conjugate base is what is left after an acid gives up a proton. The stronger the acid, the weaker the conjugate base. The weaker the acid, the stronger the conjugate base.
Fill in the blanks in the table below.
| Acid | Base | Equation | |
| 1. | H2SO4 | HSO4- | H2SO4H+ + HSO4- |
| 2. | H3PO4 | ||
| 3. | F- | ||
| 4. | NO3- | ||
| 5. | H2PO4 | ||
| 6. | H2O | ||
| 7. | SO4-2 | ||
| 8. | HPO4-2 | ||
| 9. | NH4+ | ||
| 10. | H2O |
Q6)Given the following solutions:
Solution A: pH of 10 Solution B: pH of 7 Solution C: pH of 5
Which list has the solutions placed in order of increasing H+ concentration?
- A, B, C 2. B, A, C 3.C, A, B 4. C, B, A
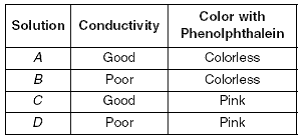
Q7)A student was given four unknown solutions. Each solution was checked for conductivity and tested with phenolphthalein. The results are shown in the data table below.
Based on the data table, which unknown solution could be 0.1 M NaOH?
- A 2. B 3. C 4. D
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


