Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q10) Two containers thermally isolated from the environment contain the same amount of nitrogen gas. They are initially connected with a closed valve. If
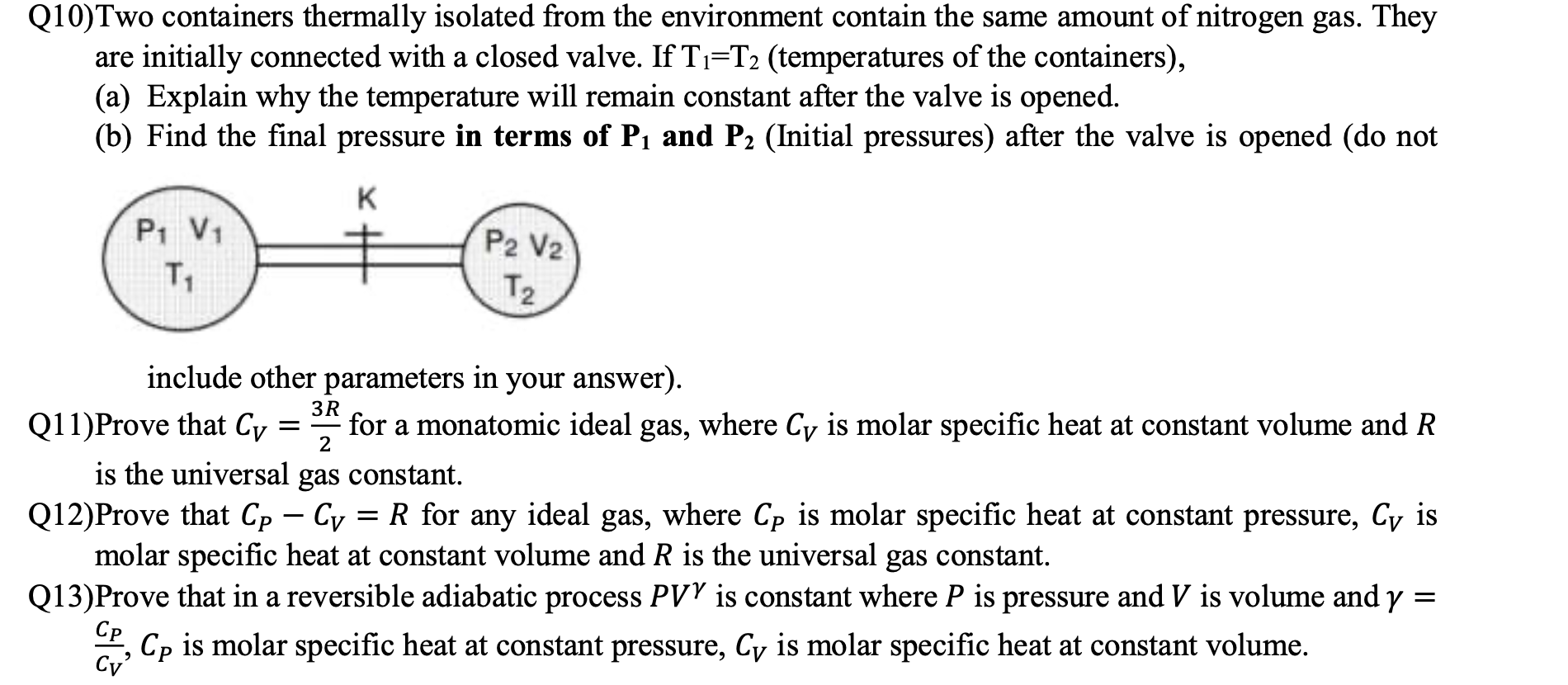
Q10) Two containers thermally isolated from the environment contain the same amount of nitrogen gas. They are initially connected with a closed valve. If T-T2 (temperatures of the containers), (a) Explain why the temperature will remain constant after the valve is opened. (b) Find the final pressure in terms of P and P (Initial pressures) after the valve is opened (do not K P V T include other parameters in your answer). P2 V2 T 3R 2 is the universal gas constant. Q12)Prove that Cp Cy = R for any ideal gas, where Cp is molar specific heat at constant pressure, Cy is molar specific heat at constant volume and R is the universal gas constant. = Q13)Prove that in a reversible adiabatic process PVY is constant where P is pressure and V is volume and y Cp is molar specific heat at constant pressure, Cy is molar specific heat at constant volume. Cp Cv' Q11) Prove that Cy = for a monatomic ideal gas, where Cy is molar specific heat at constant volume and R
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer of 10th question To answer this question we need to consider the principles of thermodynamics and the behavior of gases a Explanation for the temperature remaining constant after the valve is o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


